3723
Biochemical and behavioral alterations in a ferret model of blast related mild traumatic brain injury1Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Center for Advanced Imaging Research (CAIR), University of Maryland School of Medicine, Baltimore, MD, United States, 3Blast Induced Neurotrauma Branch, Walter Reed Army Institute of Research, Silver Spring, MD, United States, 4The Geneva Foundation, Tacoma, WA, United States
Synopsis
We studied the longitudinal changes in brain metabolism and impulsivity behavior in ferrets, a gyrencephalic animal model subjected to novel blast exposure conditions that closely mimic those encountered by Warfighters. Compared to rodents, the ferret model has greater similarities to human in terms of developmental process, brain structure and sophisticated behavior. Ferrets demonstrated increased behavioral impulsivity and higher glutamate and taurine in prefrontal cortex following blast exposure. Our findings are in agreement with clinical observations in patients, suggesting that this model is a good gyrencephalic animal model to study brain biochemical profile changes and neuropsychiatric alterations associated with blast exposure.
Introduction
Rodent animal models have been widely used to assess neurobiological underpinnings and therapeutic interventions in traumatic brain injury (TBI). Despite the valuable insights these models have provided, there is a growing interest in identifying models of other species with neuroanatomical features closely resembling human brains. With this purpose, we established a gyrencephalic model of blast (ferret) which underwent blast exposure that closely mimic the conditions that are experienced by Warfighter 1. In this study, we performed in vivo longitudinal magnetic resonance imaging (MRS) and behavioral assessment to test whether biochemical and behavioral profile after blast exposure.Methods
Animal procedureFitch ferrets aged around postnatal day 100 were anesthetized with 4% isoflurane for 6 minutes and exposed to a blast overpressure of ~140 KPa with 4-5 msec positive pressure duration using an advanced blast simulator that simulates "free-field" blast that are experienced by Warfighter 2. This generates a model of mild blast TBI. Sham group underwent the anesthesia for the same duration without receiving the blast overpressure. Six blast ferrets and five sham ferrets were included in this study. Imaging experiments were performed at 3-day, 7-day, 1-month and 3-month post-injury. Behavioral assessments were conducted at 1- and 3-month post-injury.
Imaging experiments
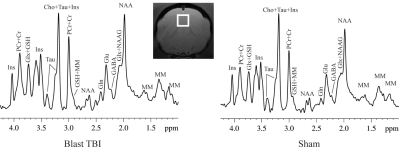
MR experiments were performed on a Bruker Biospec 7T scanner (Bruker Biospin MRI GmbH, Germany). Anesthesia was maintained with about 3% isoflurane during the imaging session. A MR compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor animal respiration rate and body temperature which was maintained at 37-38.5°C using a warm water bath circulation. 1H-MRS data were obtained from a voxel (3.5 × 3.5 × 3.5 mm3) that covered the medial prefrontal cortex (mPFC) (Figure 1). A short-TE point-resolved spectroscopy pulse sequence (TR/ TE = 2,500/10 ms, NA = 360) was used for MRS data acquisition 3. The unsuppressed water signal from the prescribed voxel was used as a reference for determining the specific metabolite concentrations. 1H-MRS data were fitted using the LCModel package (version 6.3-0G; LCModel Inc., Oakville, ON, Canada) 4. A threshold of the Cramer-Rao lower bounds ≤35 % was used to select metabolite concentrations that had acceptable level of quantification reliability 5.
Behavioral assessment
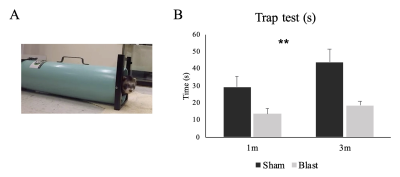
The behavioral assessment performed in this study was an in-house developed trap test for ferret. The test used a modified skunk trap with a panel to control the opening and closing of the trap at one end (Figure 2A). The animal was put inside the trap and allowed to acclimate for 2 min. After the acclimation, the panel was opened and the time that the animal took to walk out of the trap (rear feet out) was recorded. The test was designed to measure the level of anxiety and impulsivity.
Data analysis
Repeated measures ANOVA was performed on imaging and behavioral measures with group as between subject effect and visit as within subject effect. Interaction effect between group and visit was also considered.
Results
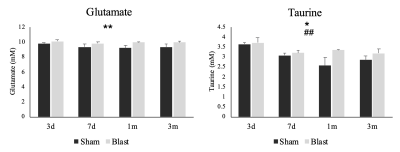
A significant main effect of group was detected for the time the animals spent in the trap test (p=0.001) (Figure 2B). The blast ferrets spent less time to exit the trap than the sham ferrets at both 1- and 3-month post-injury. MRS data showed a significant group main effect for the glutamate (p=0.004) and taurine (p=0.036) in the mPFC. Both metabolites increased significantly in the blast ferrets, especially at 1- and 3-month post-injury. In addition, a significant main effect of visit was observed on the level of taurine in the mPFC, with the levels of taurine reducing overtime (p=0.009) (Figure 3). No significant group x visit interaction was detected.Discussion
In the trap test, the blast ferrets spent less time observing the surrounding environment and exited the opening faster than the sham ferrets. This indicated increased impulsive decision making which can be associated with prefrontal cortex-amygdular axis dysfunction following blast induced brain injury. Our findings of increased glutamate is consistent with earlier reports during acute and subacute stages following TBI which was associated with excitotoxicity and the cognitive dysfunctions 6. Increased levels of taurine, an inhibitory neuromodulator, could be the result of a compensatory mechanism to protect the brain from excessive glutamate 7. Enhanced level of taurine and glutamate in prefrontal cortex was observed in schizophrenia, bipolar disorder and major depression 8-10. Considering that TBI is a risk factor of schizophrenia 11 and psychotic disorders 12, our MRS results, together with the observed increased impulsivity, suggest a dysfunction of prefrontal cortex and potential secondary psychiatric disorders following mild blast TBI.Conclusion
In this study, we demonstrated increased behavioral impulsivity and enhanced level of glutamate and taurine in the prefrontal cortex. Our imaging findings agree with the behavioral result, indicating disrupted functioning of the prefrontal cortex which could lead to TBI induced psychiatric disorders.Acknowledgements
This study was supported by Walter Reed Army Institute of Research, Military Operational Medicine Research Program UFR: project #21630.References
1. Shively SB, Horkayne-Szakaly I, Jones RV, et al. Characterisation of interface astroglial scarring in the human brain after blast exposure: a post-mortem case series. Lancet Neurol. 2016 Aug;15(9):944-953.
2. Sajja VS, Peethambaran A., Van Albert S, et al. Rodent Model of Primary Blast-Induced Traumatic Brain Injury: Guidelines to Blast Methodology In Pre-Clinical and Clinical Methods in Brain Trauma Research, 2018. Springer, NY.
3. Xu S, Ji Y, Chen X, et al. In vivo high-resolution localized (1)H MR spectroscopy in the awake rat brain at 7 T. Magn Reson Med. 2013 Apr;69(4): 937–43.
4. Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR Biomed. 2001 Jun;14(4):260–4.
5. Morgan JJ, Kleven GA, Tulbert CD, et al. Longitudinal 1H MRS of rat forebrain from infancy to adulthood re- veals adolescence as a distinctive phase of neurometabolite development. NMR Biomed. 2013 Jun;26(6):683–91.
6. Guerriero RM, Giza CC, Rotenberg A. Glutamate and GABA imbalance following traumatic brain injury. Curr Neurol Neurosci Rep. 2015 May;15(5):27.
7. Wu H, Jin Y, Wei J, et al. Mode of action of taurine as a neuroprotector. Brain Res. 2005 Mar 21;1038(2):123-31.
8. Shirayama Y, Obata T, Matsuzawa D, et al. Specific metabolites in the medial prefrontal cortex are associated with the neurocognitive deficits in schizophrenia: a preliminary study. Neuroimage. 2010 Feb 1;49(3):2783-90.
9. Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007 Dec 1;62(11):1310-6.
10. van Elst LT, Valerius G, Büchert M, et al. Increased prefrontal and hippocampal glutamate concentration in schizophrenia: evidence from a magnetic resonance spectroscopy study. Biol Psychiatry. 2005 Nov 1;58(9):724-30.
11. Molloy C, Conroy RM, Cotter DR, et al. Is traumatic brain injury a risk factor for schizophrenia? A meta-analysis of case-controlled population-based studies. Schizophr Bull. 2011 Nov;37(6):1104-10.
12. Fujii D, Fujii DC. Psychotic disorder due to traumatic brain injury: analysis of case studies in the literature. J Neuropsychiatry Clin Neurosci. 2012 Summer;24(3):278-89.
Figures