3722
Simultaneous Myelin Water Imaging and 3D 1H-MRSI Relates Myelin Degradation to Neurometabolic Changes in Mild Traumatic Brain Injury Patients1Radiology Department, Shanghai Fifth People's Hospital, Fudan University, Shanghai, China, 2School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 3Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 5Radiology Department, Tong Ren Hospital Shanghai Jiao Tong University School Medicine, Shanghai, China
Synopsis
White matter tracts are known to be highly vulnerable to traumatic axonal injury. Myelin degradation is a major contributing factor for the white matter pathology in traumatic brain injury (TBI), but its relation to neurometabolite alterations is unclear. In this study, we used the latest SPICE technology to perform simultaneous 3D high-resolution MRSI and myelin water imaging to assess the relationship between myelin degradation and neurometabolic changes in acute mild TBI (mTBI) patients. Our results showed coupled MWF and NAA reductions in occipital CC tract of the patients, which provides new insights into TAI pathology in acute mTBI.
Introduction
Traumatic brain injury (TBI) is a serious public health problem, with mild traumatic brain injury (mTBI) accounting for over 80% of all the cases worldwide. Despite the significant cognitive and behavioral deficits caused by the injury, the underlying pathophysiological mechanism of mTBI remains not fully understood. White matter tracts are highly vulnerable to axonal injury from impact-acceleration force and myelin degradation is a major contributing factor for the white matter pathology of mTBI.1 Previous studies showed myelin disruption in mTBI patients using MR-based myelin water imaging.2 The changes in myelination have been suggested to contribute to the acute metabolic imbalance post mTBI.3 However, the relationship between myelin degradation and neurometabolic alterations has not been explored in clinical settings. In this study, we used the latest SPICE technology to achieve simultaneous high-resolution myelin water imaging and 3D 1H-MRSI in mTBI patients and investigated the concurrent changes of myelin water fraction (MWF) and neurometabolites. In a single 8-min MR scan, we obtained nearly whole-brain mapping of neurometabolic signals at 2.0 x 3.0 x 3.0 mm3 nominal resolution and MWF maps at 1.0 x 1.0 x 1.9 mm3 resolution. This study was focused on the corpus callosum (CC), the most frequently identified abnormal white matter fiber bundles in mTBI. Our results showed reduced MWF in the occipital CC tract, along with the colocalized increase of myo-inositol (mI) and decrease of N-acetylaspartate (NAA) concomitantly in the patients group. These findings may provide new insight into and further understanding of traumatic axonal injury.Method
We prospectively recruited 18 mTBI patients (GCS score: 13-15; LOC < 30 min; PTA < 24 hours) and 22 age-matched healthy subjects for the study. The patients were scanned at an average of 1.25 day post injury (range 1-7 days). The MR scans were performed on a 3T MR scanner (Siemens Skyra). The scanning protocols included 3D MRSI scan using the SPICE sequence 4-6 (TR/TE = 160/1.6 ms, resolution = 2.0 × 3.0 × 3.0 mm3, FOV = 240 × 240 × 120 mm3, scan time = 8 min), 3D MPRAGE imaging (TR/TE/TI = 2400/2.13/1000 ms, resolution = 1.0×1.0×1.0 mm3, FOV = 256 mm, 192 slices) and diffusion-weighted imaging (DWI) (TR/TE = 8300/74 ms, resolution = 2×2×2 mm3, FOV = 256 mm, 75 slices, 30 directions of b = 1000s/mm and 5 B0 images was acquired). This study was approved by the IRB of Shanghai Fifth People’s Hospital, Fudan University, Shanghai, China.The spatiospectral functions of metabolites were reconstructed using a union-of-subspaces model, incorporating pre-learned spectral basis functions.5-7 Spectral quantification was done using an improved LCmodel-based algorithm that incorporated both spatial and spectral priors.8 MWF estimation was done using an improved 3-exponential model fitting algorithm that incorporated pre-learned parameter distributions and low-rank signal structures.9
The MRSI-MWF and DWI images were coregistered to T1w image using affine linear transformation. The AFQ toolbox was used to extract eight CC tracts, i.e. occipital, posterior parietal, superior parietal, motor, superior frontal, anterior frontal and orbitofrontal and temporal CC tracts.10 The procedure included whole-brain deterministic tracking using the DWI images, selection and refinement of each CC fiber tract. The mean MWF and neurometabolic levels for each tract was calculated. Statistical analyses were performed to compare the regional neurometabolic and MWF changes in the CC tracts. Independent two-sample t-test was conducted for the comparison.
Results and Discussion
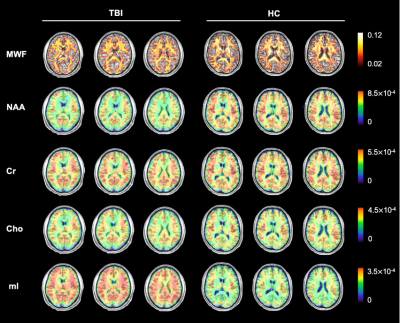
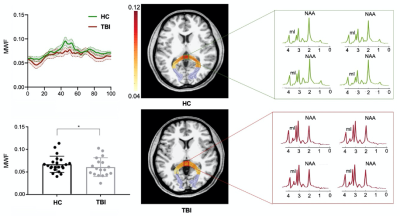
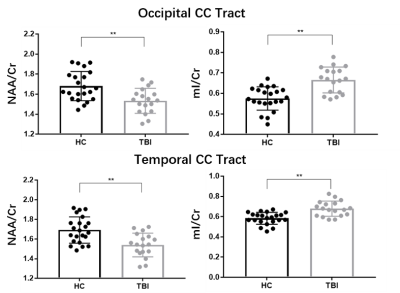
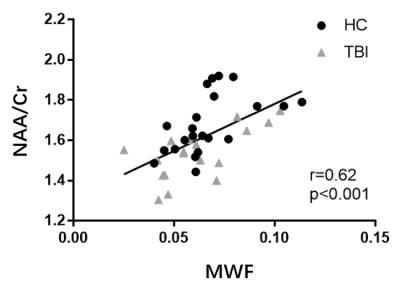
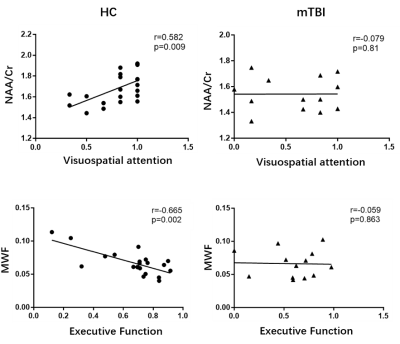
Figure 1 shows a set of representative high-resolution MWF and neurometabolic maps including NAA, mI, creatine (Cr) and choline (Cho) obtained successfully using the SPICE sequence from an mTBI patient and a healthy subject. Figure 2 shows spatially resolved spectra from the occipital tract along with group-wise colocalized MWF changes between mTBI patients and healthy controls. A reduction in MWF could be observed in the occipital tract of the patients group and the MRSI spectra revealed a reduction in NAA and an increase in mI in the patients. As shown in Figure 3, a significant group-level reduction in NAA and an increase in mI were found in the occipital and temporal tracts of CC of the patients (Bonferroni-corrected p < 0.002) were observed, in consistent with previous literature.2 The occipital CC MWF was positively correlated with NAA across both groups, as shown in Figure 4, indicating a coupled relationship between myelination and axonal integrity.11 Interestingly, we found that NAA level was positively correlated with visuospatial attention, while MWF was negatively correlated with executive function in healthy subjects occipital CC tract. Both were disrupted in the mTBI patients as shown in Figure 5.Conclusion
This study investigated the relationship between myelin degradation and neurometabolic alterations in acute mTBI patients. Our results showed concurrent neurometabolic and MWF changes in occipital CC tract of the patients. The findings may provide a foundation for further investigation to gain new insights into and further understanding of the pathophysiology underlying myelin degradation in acute mTBI using multimodal structural/metabolic imaging.Acknowledgements
Y. L. is funded by National Science Foundation of China (No.61671292 and 81871083) and Shanghai Jiao Tong University Scientific and Technological Innovation Funds (2019QYA12).References
1. Mierzwa AJ, Marion CM, Sullivan GM, et al. Components of myelin damage and repair in the progression of white matter pathology after mild traumatic brain injury. J Neuropathol Exp Neurol. 2015;74:218–32.
2. Wright AD, Jarrett M, Vavasour I, et al. Myelin water fraction is transiently reduced after a single mild traumatic brain injury- a prospective cohort study in collegiate hockey players. PLoS One. 2016;11: e0150215.
3. Giza CC, Hovda DA. The new neurometabolic cascade of concussion. Neurosurgery. 2014;75: S24-S33.
4. Lam F and Liang ZP. A subspace approach to high-resolution spectroscopic imaging. Magn Reson Med. 2014;71:1349-1357.
5. Lam F, Ma C, Clifford B, et al. High-resolution 1H-MRSI of the brain using SPICE: data acquisition and image reconstruction. Magn Reson Med. 2016;76:1059-1070.
6. Peng X, Lam F, Li Y, et al. Simultaneous QSM and metabolic imaging of the brain using SPICE. Magn Reson Med. 2018;79:13-21.
7. Ma C, Lam F, Johnson CL, et al. Removal of nuisance signals from limited and sparse 1H MRSI data using a union-of-subspaces model. Magn Reson Med. 2016;75:488-497.
8. Li Y, Lam F, Clifford B, et al, A Subspace Approach to Spectral Quantification for MR Spectroscopic Imaging. IEEE Trans Biomed Eng. 2017;64:2486-2489.
9. Li Y, Guo R, Zhao Y, et al. A Model-Based Method for Estimation of Myelin Water Fractions. Proc. Intl. Soc. Magn. Reson. Med. 2019;p.4902.
10. Yeatman JD, Wandell BA, Mezer AA. Lifespan maturation and degeneration of human brain white matter. Nat Commun. 2014;5:1-12.
11. Johnson VE, Stewart W, and Smith DH. Axonal pathology in traumatic brain injury. Exp Neurol. 2013;246: 35–43.
Figures