3714
GABA Inhibition Enhances in Epilepsy Associated with Focal Cortical Dysplasia1Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 2Philips Healthcare, Beijing, China, 3Departments of Neurology, Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China
Synopsis
The aim of this study was to investigate the metabolic dysfunction of FCD associated epilepsy using Edited MRS. 14 patients and 14 age- and sex-matched healthy controls underwent MR scans, including Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES). The results indicated that GABA levels were significantly increased in epilepsy focal region of patients compared with contralateral regions and healthy controls, while no significant alterations were found in GSH and Glx levels. GABAergic inhibition enhances in FCD foci suggested that GABA may play a central role in the pathophysiology of FCD associated epilepsy.
Introduction
Focal cortical dysplasia (FCD) is a neuronal migration disorder and is a major cause of drug-resistant epilepsy; however, the underlying mechanism for FCD metabolism in epilepsy patients remains unclear. Previous studies have indicated network metabolic dysfunction in epilepsy patients, gamma-aminobutyric acid (GABA)/glutamate as the principal inhibitory/excitatory neurotransmitter in central nervous system, and reduced glutathione (GSH) as the most abundant intracellular antioxidant may be related to the potential mechanism of FCD-associated epilepsy. However, the concentration of GABA, GSH and glutamine/glutamic acid (Glx) in the brain is too low to be reliably detected using conventional single-voxel magnetic resonance spectroscopy (MRS). The edited MRS, e.g. Mescher-Garwood Point-resolved Spectroscopy (MEGA-PRESS), was thus developed for specific detection of metabolites including GABA (1,2) , Glx and GSH (3,4). Hadamard Encoding and Reconstruction of Mega-Edited Spectroscopy (HERMES) applies multiple orthogonal editing encoding, allowing GSH, GABA and Glx spectra to be reconstructed simultaneously from one single sequence (5,6). The aim of this study is to detect the alterations of GABA, GSH and Glx in FCD-associated epilepsy using HERMES.Methods
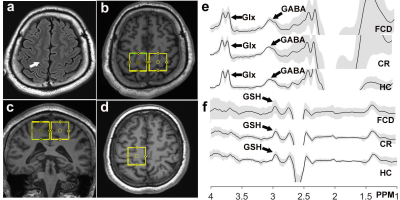
Fourteen patients who were scheduled to receive surgery for suspected diagnosis of FCD-associated epilepsy and 14 age- and sex-matched healthy controls were enrolled prospectively in this study; all subjects underwent MR scans on a 3T scanner, including 3D T1 weighted imaging and HERMES. The parameters of HERMES were as follows: TR/TE 2000/80 ms, 320 averages, voxel size 3×3×3 cm3, ~10 min per acquisition. All patients stopped taking medication at least 12 hours before the MRS data collection. The Regions of interest (ROIs) for HERMES were set at FCD foci, contralateral cerebral region (CR) and healthy control (HC), as shown in figure 1. The detected GABA, GSH and Glx signals in FCD, CR and HC were quantified using the Matlab-based (MathWorks, Natick, MA) analysis toolkit Gannet 3.1 (http://www.gabamrs.com/). The GABA signal detected by HERMES also contains co-edited signal from macromolecules and homocarnosine, so it is referred to as GABA+ below. Only spectra with a relative fitting error (FitError) of GABA generated by Gannet smaller than 15% were enrolled in the final statistical analysis. Differences of GABA+, GSH and Glx levels (adjusting for GM and WM fractions (7)) among the three groups were analyzed using analysis of covariance (ANOVA), if significant, the pairwise comparisons would be analyzed by the least significant difference (LSD) test. Segmentation of T1-weighted images was performed using SPM 12.Results
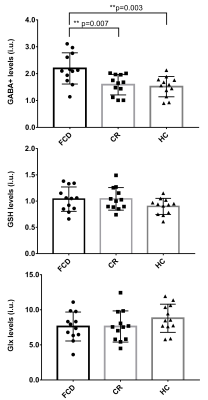
The histopathologic results indicated that one case was low-grade glioma, not a FCD foci; another MRS data was removed because of the fitting error bigger than 15% for GABA. So, 12 FCD associated epilepsy patients and 12 matched healthy controls were finally enrolled in this study. ANOVA results indicated that there is a significant difference in GABA levels among FCD foci, contralateral regions and healthy controls (F=7.97, p=0.001), while no difference was found in GSH and Glx levels, as indicated in Figure 2. The LSD results further revealed that GABA levels were significantly increased in FCD foci compared with contralateral regions (p=0.007) and with healthy controls (p=0.003).Discussion
More evidence indicated that the neurotransmitters glutamate and GABA are centrally involved in the epilepsy process. GABA is the main inhibitory neurotransmitter in the cerebral cortex, and a pioneering study has confirmed that GABA-mediated synaptic inhibition plays an essential role in the process of epileptogenesis in patients with FCD (8,9). A previous study showed that GABA levels were higher in FCD-associated epilepsy patients than controls (7), which was consistent with our results. Glutamate is primary excitatory amino acid, which was also involved in epilepsy. Slow rates of glutamate-glutamine cycling was found in epilepsy patients, inducing decreased glutamine and increased glutamate contents (10), which could account for the results of this study that no differences of Glx were found in this study. Conversely, the opposite view also exists, a previous research indicated that glutamine in epilepsy was higher than controls (7). Oxidative stress is regarded as a possible mechanism in the pathogenesis of epilepsy (11), GSH is an antioxidant, which is able to prevent damage to specific neuron caused by reactive oxygen species (ROS), GSH depletion can enhance oxidative stress, and excessive ROS may trigger the degenerative process of epilepsy (12). One previous literature (13) indicated that GSH levels were significantly reduced in the parietooccipital region of both hemispheres in epilepsy patients, while no differences were shown in epileptogenic focus and the hemisphere without epileptogenic focus. However, the GSH in this study tended to be higher in FCD associated epilepsy compared with healthy controls, even the difference was not statistically significant, which was in line with an erythrocyte GSH research (14). This discrepancy may be attributed to the differences in patient selection criteria and ROI positions, and GSH levels increased in epilepsy patients after receiving anti-epileptic drugs (15).Conclusions
HERMES was able to detect the alterations of GABA, GSH and Glx in FCD-associated epilepsy patients. GABAergic inhibition enhanced in FCD foci of epilepsy patients, while no significant alteration of GSH and Glx levels was found in this study. The results indicated that GABA may play a central role in the pathophysiology of FCD-associated epilepsy.Acknowledgements
This study was financially supported by Natural Science Foundation of Shandong (Grant No. ZR201911120706).References
1. Gong T, Xiang Y, Saleh MG, et al. Inhibitory motor dysfunction in parkinson's disease subtypes. Journal of magnetic resonance imaging : JMRI 2018;47(6):1610-1615.
2. Mescher M, Merkle H, Kirsch J, Garwood M, Gruetter R. Simultaneous in vivo spectral editing and water suppression. NMR in biomedicine 1998;11(6):266-272.
3. Sanaei Nezhad F, Anton A, Parkes LM, Deakin B, Williams SR. Quantification of glutathione in the human brain by MR spectroscopy at 3 Tesla: Comparison of PRESS and MEGA-PRESS. Magnetic resonance in medicine 2017;78(4):1257-1266.
4. Dhamala E, Abdelkefi I, Nguyen M, Hennessy TJ, Nadeau H, Near J. Validation of in vivo MRS measures of metabolite concentrations in the human brain. NMR in biomedicine 2019;32(3):e4058.
5. Saleh MG, Oeltzschner G, Chan KL, et al. Simultaneous edited MRS of GABA and glutathione. NeuroImage 2016;142:576-582.
6. Chan KL, Oeltzschner G, Saleh MG, Edden RAE, Barker PB. Simultaneous editing of GABA and GSH with Hadamard-encoded MR spectroscopic imaging. Magnetic resonance in medicine 2019.
7. Chowdhury FA, O'Gorman RL, Nashef L, et al. Investigation of glutamine and GABA levels in patients with idiopathic generalized epilepsy using MEGAPRESS. J Magn Reson Imaging 2015;41(3):694-699.
8. Calcagnotto ME, Paredes MF, Tihan T, Barbaro NM, Baraban SC. Dysfunction of synaptic inhibition in epilepsy associated with focal cortical dysplasia. J Neurosci 2005;25(42):9649-9657.
9. Khazipov R. GABAergic Synchronization in Epilepsy. Cold Spring Harb Perspect Med 2016;6(2):a022764.
10. Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia 2002;43(7):703-710.
11. Chang SJ, Yu BC. Mitochondrial matters of the brain: mitochondrial dysfunction and oxidative status in epilepsy. J Bioenerg Biomembr 2010;42(6):457-459.
12. Huusko N, Romer C, Ndode-Ekane XE, Lukasiuk K, Pitkanen A. Loss of hippocampal interneurons and epileptogenesis: a comparison of two animal models of acquired epilepsy. Brain Struct Funct 2015;220(1):153-191.
13. Mueller SG, Trabesinger AH, Boesiger P, Wieser HG. Brain glutathione levels in patients with epilepsy measured by in vivo (1)H-MRS. Neurology 2001;57(8):1422-1427.
14. Menon B, Ramalingam K, Kumar RV. Low plasma antioxidant status in patients with epilepsy and the role of antiepileptic drugs on oxidative stress. Ann Indian Acad Neurol 2014;17(4):398-404.
15. Isik M, Demir Y, Kirici M, Demir R, Simsek F, Beydemir S. Changes in the anti-oxidant system in adult epilepsy patients receiving anti-epileptic drugs. Arch Physiol Biochem 2015;121(3):97-102.
Figures