3694
Insignificant contribution of blood to NOE(-1.6)1Vanderbilt University Medical Center, Nashville, TN, United States
Synopsis
We evaluated the contribution of blood to the NOE(-1.6) from rat brains by using two blood suppression approaches: (1) signal acquisition with a diffusion-weighting of b=400s/mm2; (2) intravascular injection of 5mg/kg MION, and by measurements on an ex vivo blood sample. Results show that the NOE(-1.6) does not change significantly with diffusion weighting and is relatively weaker in ex vivo blood than that in brain, but decreases significantly in vivo after injection of MION. This study suggests that NOE(-1.6) is not mainly from blood, and that MION particles alter the NOE(-1.6) but have much weaker effects on other CEST effects.
PURPOSE
A relayed nuclear Overhauser enhancement (rNOE) saturation transfer effect at around -1.6 ppm from water, termed NOE(-1.6), was previously reported in rat and human brain1. Recently, we found that the NOE(-1.6) signal from rat brain changes significantly with intake of different gases (i.e. air, O2, and N2O), suggesting that the NOE(-1.6) signal may be related to cerebrovascular changes2. Two other publications also indicated that the NOE(-1.6) signal may include significant contributions from blood3,4. In this work, we evaluated the contribution of blood to the NOE(-1.6) signal using two blood suppression approaches and ex vivo blood samples.METHODS
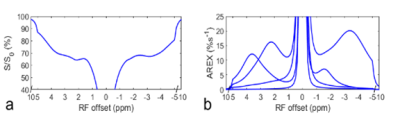
All measurements were performed on a pre-clinical Varian 9.4T MRI. Chemical exchange saturation transfer (CEST) measurements were obtained by applying a continuous wave CEST sequence with a 5s irradiation pulse followed by the SE-EPI readout. Z-spectra were acquired with RF offsets from -10 to 10ppm and an RF power of 1µT. Control signals (S0) were acquired without RF irradiation. Five rats were anesthetized with 2% isoflurane and 98% O2 for both induction and maintenance. To suppress the intravascular signal, two approaches were compared: (1) signal acquisition with a diffusion-weighting of b = 400s/mm2 to reduce intravoxel incoherent motion (IVIM) effects; (2) intravascular injection of 5mg/kg monocrystalline iron oxide nanoparticle (MION) to reduce intravascular signals. In vitro blood samples were collected using a syringe containing lyophilized heparin, and moved quickly to a sealed tube. All animal procedures were approved by the Animal Care and Use Committee of Vanderbilt University Medical Center. A multiple-pool (water, amide at 3.5ppm, amine at 2ppm, NOE at -1.6 and -3.5ppm, and semi-solid MT at -2.3ppm) Lorentzian fitting of each Z-spectrum was performed to isolate each peak. We used apparent exchange-dependent relaxation (AREX) metrics to specifically quantify NOE and CEST effects.RESULTS
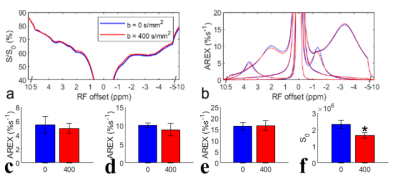
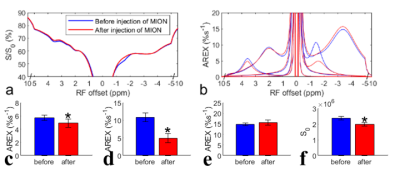
Fig. 1 shows the results of the in vivo experiment with diffusion weighting. Note that although there are significant differences between the control signals (Fig. 1f) acquired with/without diffusion weighting, due to the loss of intravascular signals and diffusion effect, there are no statistical differences between the AREX values of the CEST and NOE data acquired with/without diffusion weighting (Fig. 1c-1e). Fig. 2 shows results of the ex vivo experiment on blood. Note that the fitted NOE(-1.6) from ex vivo blood (7.0%) is lower than that from the in vivo experiments (10.1% ± 0.6%, b = 0s/mm2 in Fig. 1d). Fig. 3 shows results of the in vivo experiment acquired before/after injection of MION. The control signals (Fig. 3f) decrease significantly, indicating successful injection of MION and increased transverse relaxation. Note that although the fitted NOE(-1.6) (Fig. 3d) decreases significantly by 54.6%, the NOE(-3.5) has no significant changes and the amide signal only decreases significantly by 14.6%.DISCUSSION AND CONCLUSION
Fig. 1d also suggests a decrease of the mean AREX value for NOE(-1.6) with diffusion weighting, although it is not statistically significant. However, it only deceases by 11.9 % (from 10.1 %s-1 to 8.9 %s-1). Considering the relatively small volume of blood in the brain, the in vivo experiments with diffusion weighting and the ex vivo experiments both suggest that the NOE(-1.6) is not mainly from the blood. MION particles in flowing blood can not only suppress intravascular and perivascular signals, but add a fluctuating field to brain parenchyma which may decrease the NOE dipolar correlation time and thus decrease the NOE effect.Acknowledgements
No acknowledgement found.References
1. Zhang X, et al. Magn Reson Imaging 2016;34:1100-1106.
2 Zu Z. Magn Reson Med 2019;81:208-219.
3. Zaiss M, et al. Neuroimage 2018;179:144-155; 4. Shah SM et al. Neuroimage 2018;167:31-40.
Figures