3679
Functional evaluation of placenta with fetal growth restriction based on mDixon-Quant imaging1Lanzhou University second hospital, Lanzhou, China, 2Philips Healthcare, Guangzhou, China, 3Philips Healthcare, Xi'an, China
Synopsis
Estimating placental function is highly desirable, as impaired placental function is associated with FGR and poor neonatal outcome. This study evaluated variations of T2* after oxygen inhalation of 8 pregnant women in the second and third trimesters using quantitative mDixon-Quant imaging. The hyperoxic T2* after oxygen inhalation was significantly higher than baseline T2* which defined as before oxygen inhalation. The change of baseline T2* and hyperoxic T2* was significantly negative correlated with gestational age. The result showed that |△T2*| was correlated with placental function. We speculate T2* based mDixon-Quant imaging is useful in noninvasive assessment of placental function.
Introduction
Impairment of placental function increases the risk of fetal growth restriction (FGR). FGR is easy to cause fetal hypoxia, postpartum death and cerebral palsy and other related diseases. However, due to the special situation of gestational woman, it is difficult to evaluate placental function comprehensively. Previous studies focus on morphology rather than function of placenta by using Ultrasound[1,2]. As a noninvasive radiation-free examination with high resolution and contrast, magnetic resonance imaging (MRI) can provide a complementary information to assess the function of the placenta[3]. The aim of this study is to use mDixon-Quant imaging based transverse relaxation time (T2*) to estimate placental condition in normal pregnancies and in pregnancies complicated by FGR. Our hypothesis is that both normal pregnancy and pregnancy with FGR will have higher T2* after oxygen inhalation. And compared with FGR, normal pregnancy will have higher T2*.Methods
Eight pregnant women (5 normal pregnancies, 3 pregnancies with FGR) were included in this prospective study from July to December 2020. All subjects underwent MRI examination with a 3.0T scanner (Ingenia CX, Philips Healthcare, the Netherlands). mDixon-Quant imaging was performed before and after breathing 60% oxygen (7 L/min) via a face mask for 7 minutes, respectively. Regions of interest (ROIs) were drawn at three slices which covering the entire placenta by two independent radiologist whom had over 8 years of experience. To compare the ability of the placental oxygen transport, we defined unit gestational age T2* as |△T2*| = {(hyperoxic T2*- baseline T2*)/Gestational age}. Finally, independent two sample t-tests were used to detect changes of T2* between two groups . P-value < 0.05 was considered as statistically significant.Results
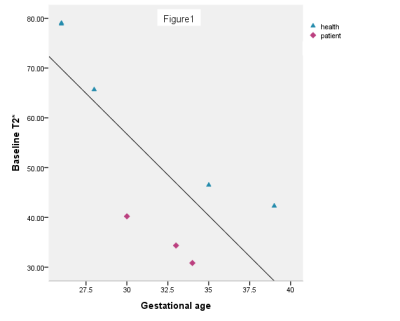
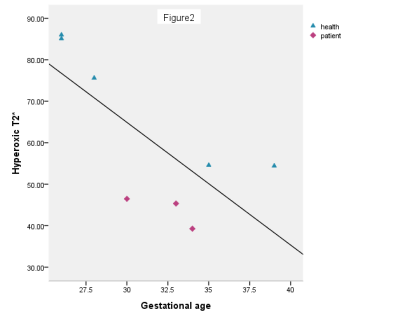
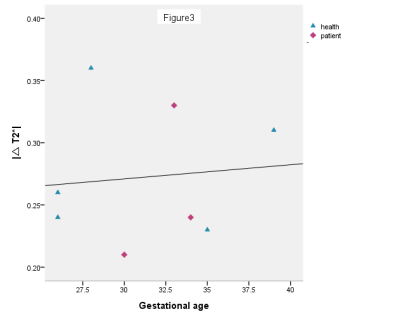
The result shows baseline T2* and hyperoxic T2* decreased with gestational age, see Figure 1 and 2. Compared with the control group, the baseline T2* and hyperoxic T2* were lower in the pregnancies with FGR (P<0.05). The |△T2*| was no difference between the two groups (P>0.05), see Figure 3.Discussion
The placenta matures and the oxygen transport capacity decreases gradually during whole pregnancy. The baseline T2* and hyperoxic T2* decreased with the increase of gestational age. In addition, the hyperoxic T2* is higher than baseline T2* both in normal and FGR pregnancy. A possible explaination is that the increment of oxygen inhaled leads to increased newly bound oxygenated hemoglobin in the placenta [4]. This study demonstrated that the baseline T2* and hyperoxic T2* of placenta are lower in pregnancies accompanied by FGR than normal pregnancies. It may be caused by the decrease of the oxygen transport capacity in the FGR placenta, which has few villi , blood vessels stenosis or even occlusion. Although there was no significant difference in the |△T2*| between the two groups, we found that the |△T2*| in normal pregnancies is higher than pregnancies with FGR. This may be caused by the small sample size and the control group has large individual difference. Despite this, we speculate that the |△T2*| in normal pregnancies is higher than pregnancies with FGR. Further studies will include a large sample size to get the consistent conclusion.Conclusion
Our study utilized mDIXON-Quant imaging to provide the pathophysiological information of placenta in pregnancy. This could be used as an objective and quantitative method to evaluate the placental function hence offering a timely adjustment for treatment scheme with dysfunction placenta.Acknowledgements
No acknowledgement found.References
1. Baschat, A. A., Neurodevelopment following fetal growth restriction and its relationship with antepartum parameters of placental dysfunction. Ultrasound Obstet. Gynecol. 2011, 37:501–514.
2. Srensen A , Hutter J , Seed M , et al., T2*‐weighted placental MRI: basic research tool or emerging clinical test for placental dysfunction?. Ultrasound in Obstetrics & Gynecology, 2020, 55(3):293-302.
3. Bernstein I. M., J. D. Horbar, G. J. Badger, et al., Morbidity and mortality among very-low-birth-weight neonates with intrauterine growth restriction. The Vermont Oxford Network. Am. J. Obstet. Gynecol. 2000, 182:198–206.
4. S. Aimot-Macron, L.J. Salomon, B. Deloison, et al. In vivo MRI assessment of placental and foetal oxygenation changes in a rat model of growth restriction using blood oxygen level-dependent (BOLD) magnetic resonance imaging. Eur. Radiol. 2013, 23:1335e1342.
5. Ingram E , Morris D , Naish J , et al., MR Imaging Measurements of Altered Placental Oxygenation in Pregnancies Complicated by Fetal Growth Restriction. Radiology, 2017, 285(3):162385.