3674
Neurite Orientation Dispersion and Density Imaging in Evaluation of Glioma-induced Corticospinal Tract Injury1Fujian Medical University Union Hospital, Fuzhou, China, 2Fujian Medical University, Fuzhou, China, 3MR Scientific Marketing, Siemens Healthcare, Shanghai, China
Synopsis
This study found that NODDI seems to be a more potent approach in evaluating the early CST infiltration by HGG, and can evaluate the CST destruction with a similar performance to MD by providing additional information about neurite density for HGG-induced CST injury.
Objective
High-grade glioma (HGG) located in or adjacent to the corticospinal tract (CST) usually involves the fiber bundle of the CST, leading to decrease of muscle strength. In order to minimize surgical damage to the CST, it is crucial to provide the neurosurgeons with accurate imaging information about CST involvement preoperatively.Recent developments in diffusion MRI have addressed some of the limitations of standard DTI and advanced capacity to characterise tissue microstructure. Newly developed neurite orientation dispersion and density imaging (NODDI) is an advanced diffusion technique that enables inference and quantification of the direction and structure of neurites (axons and dendrites) and provides valuable insights about tumour physiology.
However, up to date, no studies have applied NODDI in predicting the HGG-induced CST injury. Therefore, this study aims to apply NODDI to predict the HGG-induced CST injury, which may provide new insight about tumour physiology of HGG to the CST.
Materials and Methods
Twenty-one patients with high-grade glioma (HGG) in or adjacent to the CST pathway and 12 matched healthy subjects underwent structural and diffusion MRI.All image acquisitions were conducted on a 3T MR scanner (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) with a 64-channel receive-only head coil. The structural MR imaging protocol included pre-contrast axial fluid-attenuated inversion recovery (FLAIR) T1-weighted (T1W) images, axial T2-weighted (T2W) fast spin-echo (FSE) images, axial FLAIR T2W images, and subsequent contrast- enhanced axial/sagittal/coronal FLAIR T1W images. A grid sampling scheme was adopted for the acquisition of 128 diffusion q-space samples, which consisted of 14 b-values of b = {250, 500, 750, 1000, 1250, 1500, 2000, 2250, 2500, 2750, 3000, 3250, 3500, 4000} s/mm2 along 3, 6, 4, 3, 12, 12, 6, 15, 12, 12, 4, 12, 24 and 3 directions, respectively. The other scan parameters were: TR, 3900 ms, TE, 88 ms, FOV, 230 × 230 mm2, GRAPPA, 2, slice acceleration factor, 2, number of averages, 1, voxel size, 2.5 × 2.5 × 2.5 mm3, without gap, acquisition time, 8min 44sec. Because the eddy current artifact produced by grid sampling scheme can not be corrected using eddy current correction, the bipolar pulse was used to handle eddy current at the sequence level.
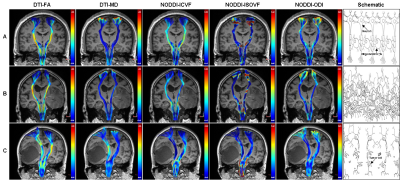
The CSTs were reconstructed on the both sides in DSI-studio (version Sep 15, 2020 build, http://dsi-studio.labsolver.org/) using generalized q-sampling imaging (GQI). The CST features including morphological features (track number, average track length and track volume) and the diffusion parameter values including fractional anisotraphy (FA), mean diffusivity (MD), intracellular volume fraction (ICVF), isotropic or free water volume fraction (ISOVF) and orientation dispersion index (ODI) along the CST were calculated. The CST features were compared between the affected and healthy side for HGG patients and between the left and right side for healthy subjects. The relative CST features were compared across the healthy subjects, patients with motor weakness and patients with normal motor function. Receiver operating characteristic (ROC) curve was applied to evaluate the performance of each relative CST characteristic for HGG-induced CST changes.
Results
Compared with the CST features on the healthy side, the track number, track volume and FA along the CST changed significantly on the affected side for HGG patients (p<0.05 for all), whereas MD and ICVF changed significantly on the affected side only for HGG patients with motor weakness (p=0.018 for both). In patients with motor weakness, the relative MD was significantly higher (p=0.001), whereas the relative FA and ICVF was significantly lower (p=0.005 and 0.001) than those in patients with normal motor function. The relative ICVF had a similar area under curve (AUC) to that of MD (AUC=0.959 and 0.969). Compared with the relative CST features in the healthy subjects, only the relative ICVF was significantly lower in HGG patients with normal motor function (p=0.024).Conclusion
NODDI seems to be a more potent approach in evaluating the early CST infiltration by HGG, and can evaluate the CST destruction with a similar performance to MD by providing additional information about neurite density for HGG-induced CST injury.Acknowledgements
This work was supported by grants from the Natural Science Foundation of Fujian Province (No. 2018J05135) and Joint Funds for the innovation of science and Technology, Fujian province (Grant number: 2017Y9024).References
1. Min ZG, Niu C, Zhang QL, Zhang M, Qian YC. Optimal Factors of Diffusion Tensor Imaging Predicting Corticospinal Tract Injury in Patients with Brain Tumors. Korean J Radiol 2017;18:844-851.
2. Zhao J, Li JB, Wang JY, Wang YL, Liu DW, Li XB, et al. Quantitative analysis of neurite orientation dispersion and density imaging in grading gliomas and detecting IDH-1 gene mutation status. Neuroimage Clin 2018;19:174-181.
3. Li SH, Jiang RF, Zhang J, Su CL, Chen XW, Zhang JX, et al. Application of Neurite Orientation Dispersion and Density Imaging in Assessing Glioma Grades and Cellular Proliferation. World Neurosurg 2019;131:e247-e254
Figures
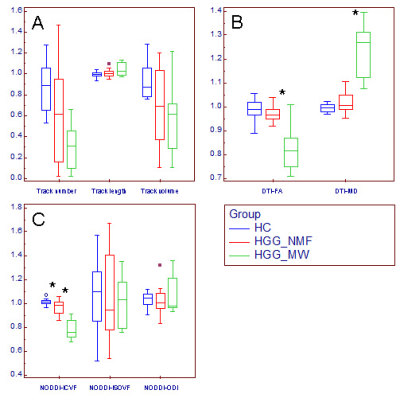
Changes of relative CST features in HGG patients. Box and whisker plot (A-C) showed that compared with the relative CST features in HGG_NMF, the relative MD was significantly higher, where the relative FA and ICVF were significantly lower (*) in HGG_MW; compared with the relative CST features in HC, the relative ICVF was significantly lower (*) in HGG_NMF; in contrast, no significant changes were found in the relative CST morphological characteristics, ISOVF or ODI.
HGG_MW = HGG patients with motor weakness, HGG_NMF = HGG patients with normal motor function, HC = healthy subject.