3671
Imaging attributes of H3K27M mutation in Diffuse Midline Gliomas on Multiparametric MRI1Neuroimaging and Interventional Radiology, NIMHANS, BENGALURU, India, 2Interventional Radiology, Paras Hospital, Gurgaon, India, 3Neuropathology, NIMHANS, BENGALURU, India, 4Neurosurgery, NIMHANS, BENGALURU, India
Synopsis
H3K27M mutant diffuse midline gliomas are a newly classified entity in the 2016 World health organization classification of CNS tumors. They are high grade tumors, with the mere presence of this mutation confers them a WHO grade IV designation, irrespective of their histologic morphology. Patients harboring these tumors have dismal prognosis and shorter overall survival. Furthermore, since these are deep-seated lesions involving the eloquent brain areas, biopsy can be challenging with substantial risk of morbidity. Our work proposes non-invasive multiparametric MRI-based imaging attributes to detect the H3K27M mutation pre-operatively. Results demonstrate a considerable accuracy on 123 patients.
Introduction
Diffuse midline glioma (DMG), H3 K27M-mutant, has been included as a new entity in the 2016 World health organization classification of CNS tumors, assembling together DIPGs and infiltrating high-grade glial tumors of the midline, carrying the similar canonical mutation at the Lysine 27 residue of the N-terminal tail of histone H31-3. The tumors harboring this mutation are high-grade lesions that are clinically aggressive and carry an unfavorable outcome compared with their wild type counterparts1,4. The deep location and involvement of eloquent areas often preclude biopsy and meaningful surgical resection of many of these tumors. As H3K27M mutation has been shown to be an important prognostic factor, it is worthwhile to noninvasively predict the H3K27M mutation status with MR imaging prior to biopsy or surgery. Therefore, our study was aimed at analyzing the conventional and advanced MRI features that might aid in discriminating the H3K27M mutant DMGs from non-mutant/wild-type (WT) DMGs.Methods
MRI features of 123 patients having DMGs (mutant: n=61, age=24.13+13.13, M/F=28/33; WT: n=62, age=35.79+18.74, M/F:33/29) were evaluated retrospectively after Institutional Ethics Committee approval. These patients had undergone either surgical resection or stereotactic biopsy. Immunohistochemistry (IHC) was performed using the Ventana Benchmark automated staining system (Ventana Benchmark-XT) to detect the H3K27M mutation. Formalin-fixed paraffin-embedded sections (4 µm) from the blocks were collected on Silane coated slides. Briefly, the sections were subjected to antigen retrieval followed by incubation with primary and then secondary antibody. The antibody used for identifying the mutation was H3K27me3 (Millipore, 07-449; 1:100) (H3.3K27Mme3, Medaysis, RM192, 1:100). Patients were scanned on 3TPhilips and 1.5TSiemens scanners. Conventional MRI features were evaluated based on VASARI (Visually AcceSAble Rembrandt Images) dataset and Intra Tumoral Susceptibility Signal (ITSS) score. Advanced MRI features based on diffusion-weighted imaging (tumoral ADC, peritumoral (PT) ADC, normalized tumoral and PTADC, ratio of tumoral and PTADC and fractional anisotropy (FA)) as well as T2* perfusion-weighted imaging (rCBV, rCBF, normalized rCBV, normalized rCBF, uncorrected rCBV, normalized uncorrected CBV, corrected CBV and K2) were analysed. Diffusion data was available in 94 cases (48 mutant, 46 WT) and perfusion data in 64 cases (34 mutant, 30 WT). Subgroup analysis was also performed between the thalamic, brainstem, pediatric (<18 years), adult (>18 years) and histopathological grade IV mutant and WT DMGs. Statistical analysis was done using R software. Between group analysis of interval scale data was conducted using non-parametric Mann-Whitney U test. Between group analysis of nominal scale data was conducted using Chi-square test. For finding cut off of interval scale variable levels to predict histone mutation status, ROC curve analysis was conducted and best cut off found using Youden’s method. P<0.05 was considered statistically significant.Results
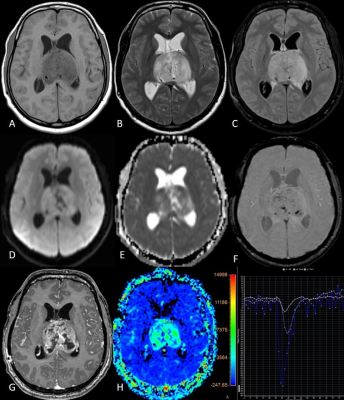
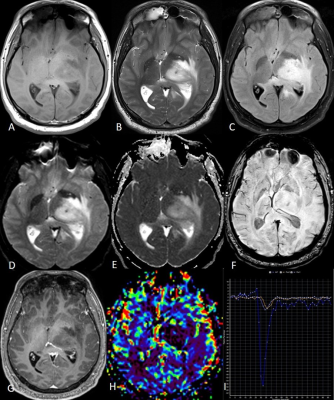
The mutant DMG group patients were younger than the WT group whereas there was no difference in the gender distribution. Five of the various conventional MRI features evaluated showed significant difference- the enhancement quality (P=0.032), thickness of the enhancing margin (P=0.05), proportion of edema (P=0.002), definition of non-contrast enhancing tumor (NCET) margin (P=0.001) and cortical invasion (P=0.037). The mutant DMGs showed significantly greater enhancement as well as greater thickness of enhancing margin. The WT DMGs exhibited significantly larger proportion of edema with poorly defined NCET margin and larger degree of cortical invasion. Few other parameters like tumor heterogeneity (P=0.07), tumor margin (P=0.074) and definition of CET margin (P=0.073) tend to approach the level of significance with a higher trend of heterogeneity in the mutant group and WT tumors showing more ill-defined margins. Among thalamic subgroup, WT DMGs showed presence of exophytic component significantly higher than the mutant DMGs. ITSS score was not found to be significantly different among various groups and subgroups. Among diffusion parameters, the peritumoral (PT) ADC and normalized PTADC (mutant: 1.64x10-3/ WT:1.83x10-3 mm2/s) were found to be significantly higher in the WT group (P=0.033 and 0.040 respectively). The thalamic subgroup showed significantly lower tumor ADC as well for the mutant DMGs (P=0.036). Among perfusion parameters, the rCBV (25.17±27.76 vs. 13.73± 14.83, P=0.018), rCBF (266.15±189.26 vs. 181.91±167.97, P=0.017) and normalized uncorrected rCBV (3.5±2.05 vs. 2.54±1.56, P=0.019) were significantly higher in the mutant group compared with WT group while the normalized rCBV (3.44±2.16 vs. 2.39±1.25, P=0.053) was trending towards significance. The brainstem and pediatric DMGs did not show any significant difference in terms of diffusion and perfusion parameters. Figures 1 and 2 are the representative images of mutant and WT DMG.Discussion
Our study elucidates the feasibility of multiparametric MRI in prediction of H3K27M mutation status in the DMGs. Identifying H3K27M status non-invasively from the first MRI scan is crucial for consequent tailored treatment planning and therapeutic intervention for improved outcomes. This study reports the highest number of DMG cases having imaging-IHC correlation. Also, the present study is the first study to report the significance of conventional and perfusion MRI in distinguishing the H3K27M mutant from the WT DMGs.Conclusion
Conventional and advanced MRI parameters can differentiate the H3K27M mutant from WT DMGs. For DMGs bearing different phenotypic and imaging characteristics, these findings carry an important implication for designing future trials in this specific neoplasm group.Acknowledgements
No acknowledgement found.References
1. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–820.
2. Daoud E V., Rajaram V, Cai C, Oberle RJ, Martin GR, Raisanen JM, et al. Adult brainstem gliomas with H3K27M mutation: Radiology, pathology, and prognosis. J Neuropathol Exp Neurol. 2018;77(4):302–311.
3. Lu QR, Qian L, Zhou X. Developmental origins and oncogenic pathways in malignant brain tumors. Wiley Interdiscip Rev Dev Biol. 2019;8(4):1–23.
4. Chen H, Hu W, He H, Yang Y, Wen G, Lv X. Noninvasive assessment of H3 K27M mutational status in diffuse midline gliomas by using apparent diffusion coefficient measurements. Eur J Radiol. 2019;114:152–159.