3664
Repeatability of VERDICT diffusion MRI in a model of human neuroendocrine tumour
Lukas Lundholm1, Mikael Montelius1, Oscar Jalnefjord1, Eva Forssell-Aronsson1, and Maria Ljungberg1
1Department of Radiation Physics, Institute of Clinical Sciences, Gothenburg, Sweden
1Department of Radiation Physics, Institute of Clinical Sciences, Gothenburg, Sweden
Synopsis
VERDICT dMRI allows for estimation of microstructural parameters in tumours which could facilitate planning and assessment of treatment. Due to the complexity of the model there is a risk for overfitting to data and there is hence a need to determine the reliability of the estimated parameters. A mouse model of human SI-NETs (n=5) was measured twice using the same dMRI protocol and the VERDICT model was fitted to data. Results showed an overall good repeatability of the tumour mean parameter values estimated by VERDICT. However, some local clusters of voxels showed larger differences between repeated scans.
Introduction
Non-invasive methods to estimate the tissue microstructure in tumours could guide the choice of treatment and be used to assess treatment effects. Diffusion MRI (dMRI) is a technique that allows for probing of the microstructure by making the MR signal sensitive to the motion of water molecules. By fitting mathematical models to the dMRI data, it is possible to indirectly estimate microstructural properties of the tissue. The Vascular, Extracellular, and Restricted Diffusion for Cytometry in Tumours (VERDICT) model is designed to estimate the voxel volume fractions of intracellular (fIC), vascular (fVASC) and extracellular extravascular (fEES) space, as well as the cell radius (R).1 VERDICT has shown promising results for diagnosing cancer and assessment of treatment.2,3 However, due to the complexity of the model there is a risk of overfitting and unreliable model parameter estimations. To make the model fitting more robust it is therefore common to fix some of the model parameters and to use regularization methods. It is hence important to determine the reliability of the parameters estimated by VERDICT in different tumour tissues.The aim of this study was to investigate the repeatability of VERDICT dMRI in a model of human small intestine neuroendocrine tumours (SI-NET).
Subjects and methods
MRI experiments were performed on Balb/C nude mice (n=5) with subcutaneous xenografts of human SI-NETs (GOT1-1) using a 7T preclinical MR system (Bruker, Biospec). Each animal was imaged twice using the same dMRI sequence, without moving the animal (scan protocol parameters in Table 1). Anaesthesia was maintained using 2-2.5 % isoflurane in air (Isoba vet., Schering-Plough Animal-health, Denmark) delivered via a nose cone. The VERDICT model fitting for estimation of R, fIC, fVASC, and fEES was performed using the AMICO framework.4 The BallSphere model was used for the intracellular and extracellular extravascular compartment, and the vascular compartment was explained using a model which separates blood flow from blood diffusion.5 The diffusion coefficients (D) of each compartment as well as the velocity dispersion (vd) of the blood flow were fixed to increase the robustness of the fit (DIC=1x10-9 m2/s, DEES=1.5x10-9 m2/s, DVASC=1.75x10-9 m2/s, vdVASC=0.6x10-3 m/s).To evaluate the repeatability of the method the repeatability coefficient (RPC) and intraclass correlation coefficient (ICC) were calculated for each estimated parameter. The RPC was calculated according to
$$RPC=1.96SD$$
where SD was the standard deviation of the differences between repeated measurements. The ICC and its 95% confidence interval were calculated using the ICC(A,1) formula.6
This study was approved by the Gothenburg Ethical Committee on Animal Research.
Results and discussion
An overall good agreement between repeated measurements was seen for the mean parameter values in the tumour (Figure 1). The fIC parameter showed the highest ICC (0.97) with a high lower bound of the 95% confidence interval (0.80). The fVASC and fEES parameters also showed good ICCs (0.80 and 0.86) but with poorer lower bounds of the confidence interval (0.10 and 0.13). All parameters representing volume fractions showed similar RPCs (0.04 – 0.06) suggesting that the absolute differences between repeated measurements were similar for these parameters. However, because fIC was estimated as much higher than fVASC and fEES in the studied tumours the relative differences between repeated measurements were lower for the fIC parameter, as reflected by its higher ICC. The results also showed that estimation of the R parameter may be less reliable (ICC=0.24) with expected differences of about 0.14±0.53 micrometres between repeated measurements in the studied tumour tissue.Some larger differences were seen when comparing the repeated measurements voxel wise (Figure 2). Although most colormap representations of the estimated parameters appeared similar on repeated experiments, some regional clusters of voxels within the tumours showed substantially changed values. Such regional changes within the tumour was seen for all parameters but was the most prevalent for the R parameter. There may have been some biophysical changes within the tumour tissue during the measurements which could have affected the dMRI signal and thus the parameter estimations. However, such changes are unlikely to fully explain the substantial differences observed in some regions of the VERDICT parameter colormaps.
Conclusion
Our results showed that VERDICT dMRI has an overall good repeatability in the studied tumour model for the tumour mean parameter values. However, VERDICT showed a large regional sensitivity to signal changes between scans which led to poorer voxel wise test-retest reliability of the estimated parameters. Further studies are required to test the reliability of the parameters estimated by VERDICT for different tumour tissue types, scan protocols, and model fitting methods.Acknowledgements
No acknowledgement found.References
- Panagiotaki et al., NRM in Biomed. 2014
- Johnston et al., Radiology. 2019
- Roberts et al., Sci Rep. 2020
- Bonet-Carne et al., NMR in Biomed. 2018
- Ahlgren et al., NMR in Biomed. 2016
- McGraw et al., Psychological Methods. 1996
Figures
Table 1. Scan
protocol parameters used for the repeatability measurements. The signal was
normalized to a b0-image acquired for every TE to compensate for T2 relaxation
effects
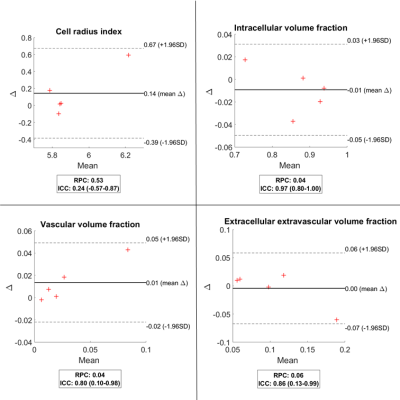
Figure 1. Bland-Altman
plots of the mean tumour parameter values for all parameters estimated with
VERDICT and all included subjects (n=5). The plots show the difference between
repeated measurements (Δ) plotted against the mean value of repeated
measurements. The repeatability coefficient (RPC) and intraclass correlation
coefficient (ICC) as well as its 95% confidence interval are shown in the small
boxes
Figure 2.
Colormaps of cell radius index (R), intracellular volume fraction (fIC),
vascular volume fraction (fVASC), and extracellular extravascular
volume fraction (fEES) as estimated by VERDICT in the central slice
of an example tumour. Colormaps are shown for two repeated measurements (M1 and
M2) and the difference between the repeated measurements (Δ)