3657
Correcting B0 inhomogeneity-induced distortions in whole-body diffusion MRI of bone metastases1Department of Radiation Medicine and Applied Sciences, UCSD School of Medicine, La Jolla, CA, United States, 2Department of Radiology, UCSD School of Medicine, La Jolla, CA, United States, 3Department of Electrical and Computer Engineering, UC San Diego, La Jolla, CA, United States, 4Department of Bioengineering, UC San Diego, La Jolla, CA, United States
Synopsis
Bone is among the most common sites of cancer metastases. Diffusion weighted imaging (DWI) can be used to detect these metastases. However, when acquired with echo-planar imaging, DWI suffers distortions due to static magnetic field inhomogeneities. In this study, we first used the reverse polarity gradient (RPG) technique to measure spatial distortions of bone metastases on DWI. Next, we demonstrated that RPG can be used to correct these distortions and produce diffusion images that more accurately reflect the underlying anatomy. Taken together, findings support the use of distortion correction techniques to improve localization of bone metastases on DWI.
Introduction
Bones are a common site of cancer metastases1, causing substantial morbidity from chronic pain, pathologic fracture, and spinal cord or nerve root compression2. Localized treatment planning and assessment of treatment response require anatomically accurate imaging. Diffusion-weighted magnetic resonance imaging (DWI) is effective for detecting bone metastases3,4, but DWI acquired with echo-planar imaging is susceptible to geometric distortions due to static magnetic field (B0) inhomogeneities5. Several techniques have been developed to measure and correct for these artifacts6–8, but none have been widely adopted for routine clinical use.In this study, we investigated whether B0 inhomogeneities led to DWI distortions that affect localization of bone metastases in whole-body DWI. We aimed to use the reverse polarity gradient (RPG) technique7 to measure the distortion in the diffusion signal of bone metastases, as well as the distortion in the diffusion signal of various skeletal landmarks. We also sought to determine whether correcting for these distortions with RPG improved anatomic correlation of DWI with T2-weighted images.
Methods
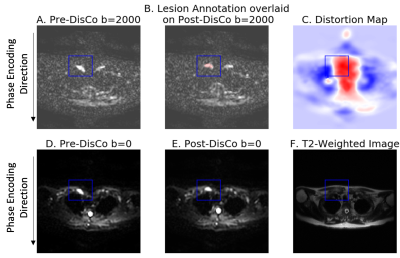
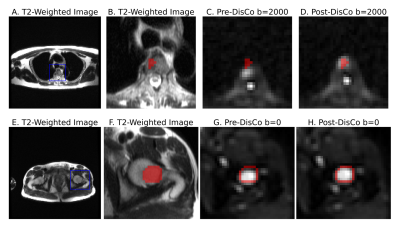
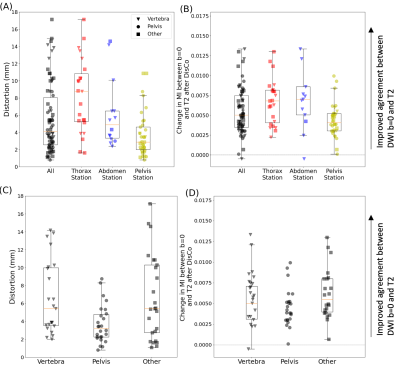
We analyzed multi-parametric whole-body MRI from 18 patients with prostate cancer bone metastases. The MRI protocol included DWI and T2-weighted imaging, conducted at five stations to cover the whole body. RPG was applied to the DWI data to generate a distortion map, where each voxel is an estimate of spatial distortion that occurred at that voxel, and to generate a distortion corrected (DisCo) DWI dataset.Lesions identified as bone metastases were annotated by a radiation oncologist and radiologist. These lesion annotations were overlaid onto the distortion maps produced by RPG. For each lesion, we selected the axial slice in which the lesion was largest. Within that slice, a rectangular bounding box was drawn around each lesion, with boundaries drawn 10 voxels from the lateral edges of the lesion. Then, the root-mean-square (RMS) was calculated from the distortion values within the box to estimate the extent to which the lesion underwent distortion (Figure 1). Next, we conducted a mutual information analysis to assess whether RPG produced diffusion images that more faithfully reflected underlying anatomy of the T2-weighted images. For each lesion, we computed normalized mutual information (MI) between the pre-DisCo DWI b=0 image and T2 image, and the normalized MI between the post-DisCo DWI b=0 image and T2 image. We compared pre-DisCo and post-DisCo MI values with Wilcoxon signed-rank tests, with each lesion representing an observation.
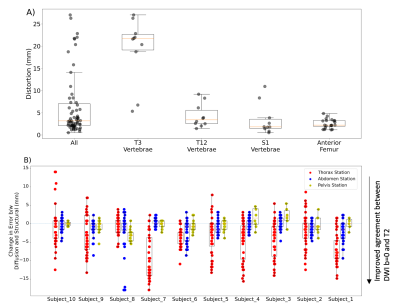
To further characterize skeletal distortions, beyond the set of bone metastases in our dataset, we annotated several landmarks across the skeleton on the structural T2-weighted MRIs. These landmarks included: most anterior point of femoral head and most posterior point of three different vertebrae (T3, T12, and S1) at a level midway between the superior and inferior edges of the vertebral body. A bounding box was drawn around each landmark with boundaries 4 voxels away in the left, right, anterior, and posterior directions, and 2 voxels away in the superior and inferior directions. For each landmark, RMS was calculated from the distortion values within the box to estimate the distortion of the landmark.
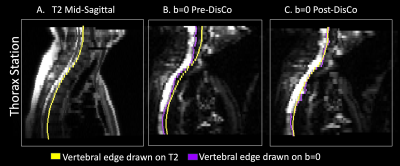
Lastly, we aimed to complement the above MI analysis with a more interpretable measure of the extent to which RPG improved similarity between diffusion and structural images. For each patient, we annotated landmarks separately on their pre-DisCo DWI b=0, post-DisCo DWI b=0, and T2 images. We again included anterior point of femoral head as a landmark. For this landmark, we calculated the distance between the pre-DisCo b=0 annotation and T2 annotation as a measure of error. We then calculated the distance between the post-DisCo b=0 annotation and T2 annotation to calculate the change in error after RPG. For the vertebrae, rather than selecting individual vertebrae, we traced the entire posterior edge of the vertebral column in the mid-sagittal plane in each imaging station and calculated changes in error at each horizontal slice.
Results
Across the entire sample, 77 individual lesions were defined and included for analysis (for lesion examples, see Figure 2). Mean (95% CI) displacement of bone lesions was 5.6 mm (95% CI: 4.8-6.5). The maximum observed displacement was 17.1 mm. Corrected diffusion images were more similar to structural MRI, as evidenced by consistent increases in mutual information after applying RPG (Wilcoxon signed-rank p<10-13) (Figure 3). Like bone metastases, our annotated femoral and vertebral skeletal landmarks also underwent substantial displacement (average, 6.3mm). Lastly, RPG led to consistent reductions in error between DWI and T2 for each of the included skeletal landmarks (mean, [95% CI]): thoracic vertebrae (-3.8 mm, [-4.3,-3.3]), abdominal vertebrae (-1.0 mm, [-1.2,-0.71]), pelvic vertebrae (-0.6 mm, [-1.0,-0.17]), and femoral head (-1.2 mm, [-2.1,-0.4]). The largest observed error reductions for thoracic, abdominal, and pelvic vertebrae, respectively, were -18.0, -18.0, and -5.5 mm (Figure 5).Discussion
We found that B0 inhomogeneity resulted in distortion of whole-body diffusion images that led to artifactual displacement of bone and bone metastases. These distortions may be severe enough to interfere with accurate biopsy or stereotactic treatment, as well as complicate interpretation of tumor shrinkage or growth. The RPG technique used here is efficient and reduced distortion artifacts in whole-body DWI. These findings support the use of distortion correction techniques to improve localization of bone metastases on DWI.Acknowledgements
The authors would like to thank David Karow, MD, PhD and Nathan White, PhD for their contributions toward establishing whole-body diffusion MRI at our institution, as well as Dominic Holland, PhD for his contributions to development of the distortion correction method applied in this work.
Grant Support: This work was supported by the NIH/NIBIB #K08EB026503, American Society for Radiation Oncology, and the Prostate Cancer Foundation. This work was further supported by the National Institute on Aging T35 grant AG26757 (PI: Dilip V. Jeste, MD, and Alison Moore, MD, MPH), and the Stein Institute for Research on Aging and the Center for Healthy Aging at the University of California, San Diego.
References
1. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584-593. doi:10.1038/nrc867
2. Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res Off J Am Assoc Cancer Res. 2006;12(20 Pt 2):6243s-6249s. doi:10.1158/1078-0432.CCR-06-0931
3. Eiber M, Holzapfel K, Ganter C, et al. Whole-body MRI including diffusion-weighted imaging (DWI) for patients with recurring prostate cancer: Technical feasibility and assessment of lesion conspicuity in DWI. J Magn Reson Imaging. 2011;33(5):1160-1170. doi:10.1002/jmri.22542
4. Jambor I, Kuisma A, Ramadan S, et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18 F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016;55(1):59-67. doi:10.3109/0284186X.2015.1027411
5. Bihan DL, Poupon C, Amadon A, Lethimonnier F. Artifacts and pitfalls in diffusion MRI. J Magn Reson Imaging. 2006;24(3):478-488. doi:10.1002/jmri.20683
6. Andersson JLR, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003;20(2):870-888. doi:10.1016/S1053-8119(03)00336-7
7. Holland D, Kuperman JM, Dale AM. Efficient correction of inhomogeneous static magnetic field-induced distortion in Echo Planar Imaging. NeuroImage. 2010;50(1):175-183. doi:10.1016/j.neuroimage.2009.11.044
8. Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65-73. doi:10.1002/mrm.1910340111
Figures