3650
Directions or Averages? An Ablation Study for in vivo Cardiac DTI1Cardiovascular Research Center, Cardiology Division, Massachusetts General Hospital, Charlestown, MA, United States, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Harvard Medical School, Boston, MA, United States
Synopsis
Recent advances in in vivo cardiac DTI have enabled rapid acquisition of the required diffusion weighted (DW) images. With the possibility of acquiring more DW images, it is not clear what is the best sampling strategy. Here we evaluate whether there are any differences between acquiring twice as many directions versus duplicating the number of repetitions. Our results indicate that it is marginally better to acquire multiple directions and that the loss of accuracy is produced by the reduction in the total number of DW images.
Introduction
Cardiac DTI has been increasingly used due to the recent introduction of second order motion-compensated diffusion weighted (DW) sequences [1–4]. These enabled the acquisition of each DW image within a single heartbeat, hence reducing the total scan time and allowing for free-breathing acquisition [5]. Despite this surge in interest, the field of cardiac DTI still lacks consensus about standard acquisition, processing and model fitting protocols. For example, it is not clear whether it is preferable to acquire more repetitions (i.e. averages) or gradient directions given a fixed scan time. In this work, we address this question with an ablation study of a cardiac DTI dataset. Specifically, we test whether there are any differences between acquiring two times more directions versus duplicating the number of repetitions.Methods
Cardiac DTI was performed on 11 healthy volunteers (mean age 43 years old, 5 female) after obtaining written consent under the appropriate approval from the institutional review board of the Massachusetts General Hospital. Scanning was performed on a 3T clinical MRI with a standard 32-channel antero-posterior surface coil. The DTI acquisition was done with M2 2DRF zoomed EPI [5] (2.5x2.5x8mm3, matrix size 150x44, pixel bandwidth 2380 Hz/pixel, 1/3 reduced FOV, FOV=375mm, Partial Fourier=6/8, TE=79ms, TR= number of slices x RR) with 30 DW images at b = 50 s/mm2 and 30 DW gradient directions x 2 averages at b = 500 s/mm2. Before fitting the DTI model, all DW images were registered to a reference at the end exhale position using affine registration. Afterwards, the b=500 s/mm2 images were grouped into 4 different datasets for the ablation study. The datasets consisted of a reference formed by all 30 directions x2 repetitions, two sets including 30 directions with a single repetition (30x1), two sets of 15 directions with 2 repetitions (15x2) and four sets of 15 directions with a single repetition (15x1). All groups included all b=50 s/mm2 images to estimate S0. The DTI model was fitted to each group using weighted-least-squares and its standard parameters –mean diffusivity (MD), fractional anisotropy (FA) and helix angle (HA)— were computed. To estimate the prediction error, the DTI model was used to simulate 15 DW images randomly selected from those excluded from the fitting and their normalized root-mean-squared error (nRMSE) was computed. Results across subjects are reported in the standard AHA format and the change of HA across the myocardium (HAT) was computed for each segment. Statistical tests were done using Wilcoxon rank test and significance level p<0.05.Results
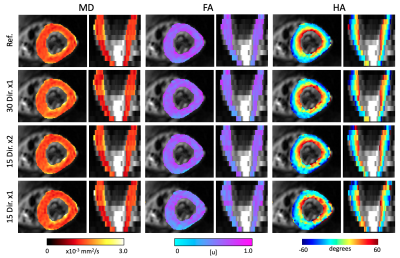
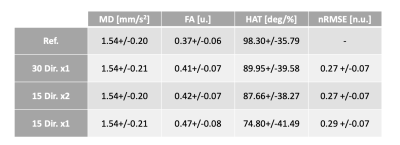
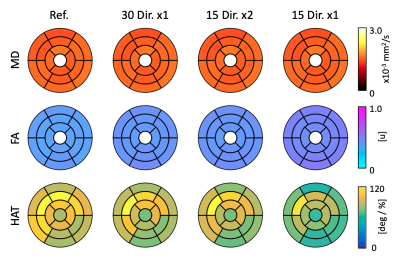
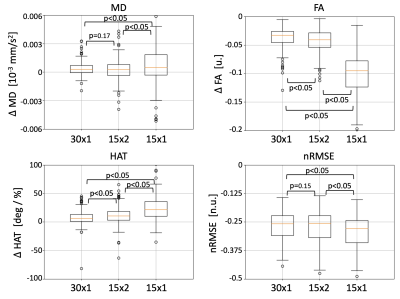
The DTI parameter maps for a representative subject are shown in Figure 1. Across datasets in the ablation study, MD remains constant and FA tends to increase with a reduction in number of gradient directions. Similarly, the HA map obtained with the 15x1 dataset presents large inhomogeneities that might disrupt the estimation of HAT. We quantify these observations in the average values of Table 1. As expected, MD remained equal across groups, FA increased slightly for 15x2 and, even more so, for 15x1. Similarly, HAT presents a large reduction between the reference and any subsequent reduction in the total number of DW images used (i.e. 30x1 and 15x2 are similar, but 15x1 has further reduced HAT). The average prediction nRMSE was equal for all datasets. The spatial distributions of these results are shown in the AHA plots of Figure 2. Both MD and FA present homogeneous spatial distributions, although FA changes across datasets. On the other hand, HAT is reduced for segments along the inferolateral free wall for 30x1 and 15x2 and throughout the entire free wall for 15x1. These errors are localized in the areas most affected by geometric distortion. Finally, we report on the error between the reference dataset and the other datasets in the ablation study. Figure 3 shows the difference between the parameter value of each subject and AHA segment obtained with the reference and the datasets 30x1, 15x2 and 15x1. As expected, the group with less DW images, 15x1, resulted in a significant increase in error for all parameters, as well as in nRMSE. On the other hand, 30x1 and 15x2 had similar values for MD and nRMSE, but increased FA and HAT error for 15x2. In all cases, the error in MD was negligible compared to its average values and the relative error in FA and HAT were less than 10% and 20% for 30x1 and 15x2.Conclusions
We evaluated the differences in gradient direction acquisition schemes for whole-heart cardiac DTI and demonstrated that acquiring DW images along different gradient directions is marginally preferable to acquiring multiple repetitions of the same directions. Moreover, reducing the number of DW images to only 15 directions with a single average results in a significant loss of accuracy in FA and HA.Acknowledgements
This work was partially funded by the National Institutes of Health awards R01HL151704 and R01HL135242.References
[1] C. T. Nguyen et al., “In vivo diffusion-tensor MRI of the human heart on a 3 tesla clinical scanner: An optimized second order (M2) motion compensated diffusion-preparation approach,” Magn. Reson. Med., vol. 76, no. 5, pp. 1354–1363, Nov. 2016.
[2] C. T. Stoeck, C. von Deuster, M. Genet, D. Atkinson, and S. Kozerke, “Second-order motion-compensated spin echo diffusion tensor imaging of the human heart,” Magn. Reson. Med., vol. 75, no. 4, pp. 1669–1676, Apr. 2016.
[3] C. L. Welsh, E. V. R. DiBella, E. W. Hsu, E. V. R. Di Bella, and E. W. Hsu, “Higher-Order Motion-Compensation for in Vivo Cardiac Diffusion Tensor Imaging in Rats,” IEEE Trans. Med. Imaging, vol. 34, no. 9, pp. 1843–1853, Sep. 2015.
[4] E. Aliotta, H. H. Wu, and D. B. Ennis, “Convex optimized diffusion encoding (CODE) gradient waveforms for minimum echo time and bulk motion-compensated diffusion-weighted MRI,” Magn. Reson. Med., vol. 77, no. 2, pp. 717–729, Feb. 2017.
[5] C. T. Nguyen et al., “Free‐breathing diffusion tensor MRI of the whole left ventricle using second‐order motion compensation and multitasking respiratory motion correction,” Magn. Reson. Med., In press Nov. 2020.
Figures