3647
Ultra-strong gradient diffusion MRI at 7T with a head insert1University Medical Center Utrecht, Utrecht, Netherlands, 2CUBRIC, Cardiff University, Cardiff, United Kingdom, 3Spinoza Centre for Neuroimaging Amsterdam, Amsterdam, Netherlands
Synopsis
Diffusion weighting is achieved by the application of external field gradients typically for tens of milliseconds, during which the signal substantially decays due to inherent T2 relaxation. This work focuses on the benefits of strong gradients - here provided by a gradient head insert - for high SNR and short TE diffusion imaging at 7T. Proof-of-principle images show that a short TE (21 ms) at a b-value of 1000 s/mm2 is achievable at 7T using an EPI-readout.
Introduction
Diffusion MRI (dMRI) relies on the application of external field gradients to achieve diffusion weighting, commonly within the context of a spin echo experiment. Such diffusion encoding gradients are typically on for tens of milliseconds, during which the signal substantially decays due to inherent T2 relaxation. The duration of diffusion encoding gradients for a given degree of diffusion weighting (commonly quantified by the b-value) can be significantly shortened by the use of strong gradients, which are becoming more readily available in body systems1,2 and gradient head inserts3,4 at 3T. The reduced echo times (TE) result in significantly higher SNR and enable the study of diffusion properties in short-T2 compartments such as myelin water5,6. In addition to stronger gradients, stronger magnetic fields can further considerably boost the SNR. However, dMRI at 7T is often considered to be challenging or even disadvantageous because of the shorter apparent T2 - i.e. the additional loss of phase coherence when spins diffuse in local susceptibility fields - which negates the SNR gain. Nonetheless, the shorter TE enabled by strong gradients provides an exciting prospect to rekindle dMRI at 7T and truly benefit from its increased SNR7, as well as enhanced susceptibility-effects indicative of myelin properties8. This work presents a proof-of-principle of ultra-strong gradient dMRI at 7T using a gradient head insert.Methods
Gradient head insertThe gradient head-insert consisted of a lightweight (45 kg) single-axis gradient coil operating in the z-direction9, which was powered by a dedicated amplifier (630A/990V Prodrive, Eindhoven). This combination yielded a maximum gradient amplitude and slew rate of 200 mT/m and 1300 T/m/s, respectively. The head insert was operated as an additional 4th gradient axis to the whole-body gradient setup and controlled via a separate gradient waveform generator.
Data
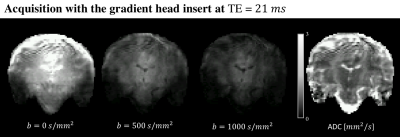
One healthy control was scanned with a dMRI acquisition protocol on 7T (Philips, NL) with and without the gradient head insert. As a proof-of-principle, the readout time was shortened by enabling partial Fourier 3/5 and parallel imaging with a SENSE factor of 2.3 for a 224x224 mm2 FOV and a 2 mm isotropic resolution. Diffusion-weighted images up to a b-value of 1000 s/mm2 were obtained with TE=21 ms using the head insert gradients. The data was corrected for susceptibility distortions with a reversed phase-encoding b=0 s/mm2 image10.
Results
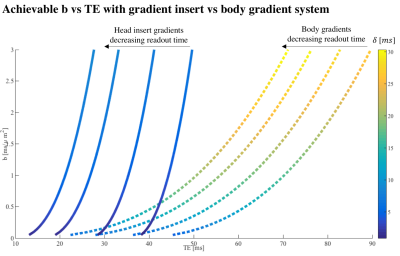
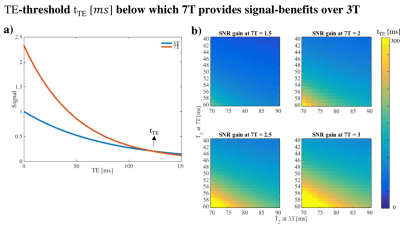
Fig.1 shows the reduced TE as a function of b-value achievable with the gradient head insert compared to 40 mT/m body gradients, for different readout times ([0, 5, 10, 15] ms). Fig.2a shows that for sufficiently short TE, the signal gain from ultra high field outweighs the more rapid signal loss from the short apparent T2, where tTE is the TE threshold for which the 3T and 7T dMRI signals are equal. Here, a linear increase in signal was assumed as a function of field strength11 and the apparent T2 was set to 50 and 77 ms at 7T and 3T for white matter respectively12. Fig.2b shows how tTE varies for varying 7T signal gains and T2 settings. Fig.3 shows images acquired at TE=21 ms with the gradient head-insert operating at 1000 T/m/s and 200 mT/m.Discussion and Conclusion
The simulation results show that using strong gradients - e.g. provided by head gradient inserts - is paramount for diffusion imaging at 7T. Here, the increased gradient performance allows for high b-values at echo times under 50 ms, which are unattainable with body gradients.Preliminary imaging results with a single-axis insert showed that TE at 21 ms for b=1000 s/mm2 was feasible at 7T using a conventional EPI-readout. Future work will focus on improving image quality, notably the signal loss in the temporal lobes originating from B1+ inhomogeneities, amplified noise due to spiking from a defect in the MR-scanner, and Gibbs ringing. B1+ inhomogeneities originate from the quadrature transmit coil currently used for the head insert. These inhomogeneities could be mitigated by replacing this coil by a multi-transmit array13. The image readout can furthermore be improved while maintaining short TE by adopting alternative strategies such as spiral readout5,14 and accelerated EPI readout enabled by the coil9.Acknowledgements
CMWT was supported by a Veni grant (17331) from the Dutch Research Council (NWO) and a Sir Henry Wellcome Fellowship (215944/Z/19/Z).References
[1] K. Setsompop, R. Kimmlingen, E. Eberlein, T. Witzel, J. Cohen-Adad, J. A. McNab,B. Keil, M. D. Tisdall, P. Hoecht, P. Dietz, S. F. Cauley, V. Tountcheva, V. Matschl, V. H.Lenz, K. Heberlein, A. Potthast, H. Thein, J. Van Horn, A. Toga, F. Schmitt, D. Lehne,B. R. Rosen, V. Wedeen, and L. L. Wald. Pushing the limits of in vivo diffusion MRI forthe Human Connectome Project. NeuroImage, 80:220–233, 2013. ISSN 10538119.
[2] D.K. Jones, D.C. Alexander, R. Bowtell, M. Cercignani, F. Dell’Acqua, D.J. McHugh, K.L.Miller, M. Palombo, G.J.M. Parker, U.S. Rudrapatna, and C.M.W. Tax. Microstructuralimaging of the human brain with a ‘super-scanner’: 10 key advantages of ultra-stronggradients for diffusion MRI.NeuroImage, may 2018. ISSN 1053-8119
[3] M. Weiger, J. Overweg, M. B. Rösler, R. Froidevaux, F. Hennel, B. J. Wilm, A. Penn, U. Sturzenegger, W. Schuth, M. Mathlener, et al. A high-performance gradient insert for rapidand short-T 2 imaging at full duty cycle. Magnetic Resonance in Medicine, 79(6):3256–326
[4] E. T. Tan, Y. Hua, E. W. Fiveland, M. E. Vermilyea, J. E. Piel, K. J.Park, V. B. Ho, and T. K.F. Foo. Peripheral nerve stimulation limits of a highamplitude and slew rate magnetic field gradient coil for neuroimaging. Magnetic Resonance in Medicine, 83(1):352–366, jan 2020
[5] L. Mueller, S. U. Rudrapatna, C. M.W. Tax, R. Wise, and D. K. Jones. Diffusion MRI with b=1000 s/mm2 at TE < 22 ms using single-shot spiral readout and ultra-strong gradients: Implications for microstructure imaging. ISMRM, page 0766, 2019.
[6] C. M.W. Tax, U. S. Rudrapatna, L. Mueller, and D. K. Jones. Characterizing diffusion of myelin water in the living human brain using ultra-strong gradients and spiral readout. ISMRM, page 1115, 2019
[7] S. Moeller, P. P. Kumar, J. Andersson, M. Akcakaya, N. Harel, R. Ma, X. Wu, E. Yacoub, C. Lenglet, and K. Ugurbil. Diffusion imaging in the post HCP era. Journal of Magnetic Resonance Imaging, 2020.
[8] E. Kleban, C. M.W. Tax, U. S. Rudrapatna, D. K. Jones, and R. Bowtell. Strong diffusion gradients allow the separation of intra- and extra-axonal gradient-echo signals in the human brain. NeuroImage, 217:116793, 2020.
[9] T. A. van der Velden, C. C. van Leeuwen, E. R. Huijing, M. Borgo, P. R. Luijten, D. W.J. Klomp, and J. C.W. Siero. A lightweight gradient insert coil for high resolution brain imaging. ISMRM, page 4329, 2017.
[10] J. L.R. Andersson, S. Skare, and J. Ashburner. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 20(2):870–888, oct 2003.
[11] O. Ocali and E. Atalar. Ultimate intrinsic signal-to-noise ratio in MRI. Magnetic resonance in medicine, 39(3):462–473, 1998.
[12] E.F Cox and P.A. Gowland. Measuring t2 and t2’ in the brain at 1.5t, 3t and 7t using ahybrid gradient echo-spin echo sequence and epi.ISMRM, page 1411, 2008.
[13] C. M. Collins, W. Liu, B. J. Swift, and M. B. Smith. Combination of optimized transmit arrays and some receive array reconstruction methods can yield homogeneous images at very high frequencies. Magnetic Resonance in Medicine, 54(6):1327–1332, dec 2005.
[14] B. J. Wilm, F. Hennel, M. B. Roesler, M. Weiger, and K. P. Pruessmann. Minimizing the echo time in diffusion imaging using spiral readouts and a head gradient system. Magnetic Resonance in Medicine, 84(6):3117–3127, 2020.
Figures