3645
Optimizing DWI b-value Sampling for Accurate Metabolic and Cytometric Parameter Extraction: Activity MRI [aMRI]1Advanced Imaging Research Center, Oregon Health & Science University, Portland, OR, United States, 2Surgery, Oregon Health & Science University, Portland, OR, United States, 3Diagnostic Radiology, Oregon Health & Science University, Portland, OR, United States
Synopsis
The optimal data acquisition strategy for accurate metabolic and cytometric parameter extraction using a digital DWI library is investigated using simulations under the constant data-acquisition time (same number of b-values) constraint. Base on DWI data from pancreatic tail tissue and using a seven b-value approach, the optimal maximum b-value is found to be data signal-to-noise ratio dependent, and generally should be below 3,000 s/mm2. In addition, evenly-spaced b-values are generally within the optimal b-spacing range.
Introduction
Diffusion-weighted MRI (DWI) is used widely in the clinic. However, its biological interpretation remains an area of active investigation. Recently, a DWI simulation library based on three metabolic and cytometric tissue parameters (homeostatic cellular water efflux rate constant [kio], cell density [ρ], and mean cell volume [V]), has been suggested and tested using Monte Carlo random walks within Voronoi-cell ensembles.1,2 Using aMRI library-simulated DWI data that matches experimental pancreatic DWI results, the goal of this work is to investigate the optimal b-value sampling strategy for accurate parameter extraction under the constant data-acquisition time (fixed number of b-values) condition.Methods
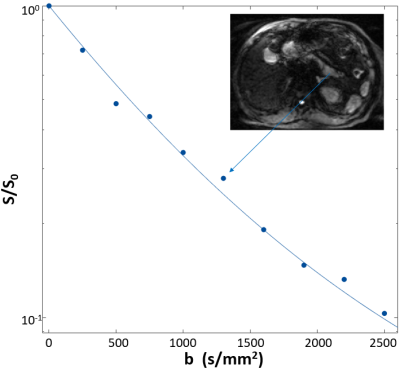
After informed consent, DWIs were acquired on normal subjects. The protocol employed thirteen b-values with 128x128 in-plane matrix, (36 cm)2 FOV, and 5.0 mm slice thickness for a total acquisition time of ~4.7 minutes. The DWI decays were first de-noised3,4 before applying library-matching to obtain the best-matched parameter set. Figure 1 shows a de-noised single-voxel DWI curve from the pancreatic tail. The inset shows the b = 750 s/mm2 DW image. The blue best-matching curve and its parameter values [kio = 71 s-1, ρ = 660,000 cells/μL, V = 1.1 pL] were then taken as “true” inputs for acquisition optimization simulations.To make the simulation closer to clinical protocols, we investigate the optimal acquisition under seven b-values (constant acquisition time). The optimal b-value extent (maximum) and distribution is investigated through Monte Carlo simulations. The primary b-value spacing employed a power‑law sampling pattern5 defined as: bi = b1 + (bn - b1) · [(i - 1) / (n - 1)]r where b1 and bn are the first and last b values; n is the total number [here, 7]; bi is the ith value [the index i runs from 1 to n]; the exponent r determines the b value spacing. Additional b spacing included common logarithmic strategies. The bn value, bmax, was varied from 2,000 to 7,000 s/mm2 for all sampling patterns.
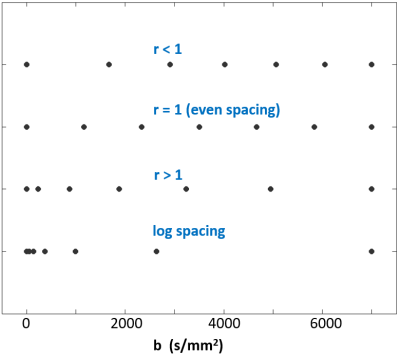
Representative spacings, with bmax = 7,000 s/mm2, are shown in Figure 2. When r < 1, spacing is larger at lower b values (i.e., sampling weighted more towards high b); when r = 1, there is even sampling; when r > 1, spacing is larger at higher b values (i.e., sampling weighted more towards initial DWI decay). The log-spacing shown at the bottom represents a different sampling strategy heavily sampled at lower b-values. For each b‑array, 2,500 simulations were run with Rician signal distribution6 produced by adding random Gaussian noise to both real and imaginary channels independently before sum-of-square signal reconstruction. Each individual set of 2,500 simulations created at different signal‑to-noise ratios (SNRs) is then reshaped to a 50x50 matrix, and the same de-noising method3,4 was applied to the simulated data. After de-noising, no appreciable deviation from Gaussian signal distribution was observed in any 2,500 samples.
Since the library approach lacks analytical methods for defining a standard error surface in a multi-variate problem, matching accuracy (a), defined as the fraction (a ≤ 1) of the matched de-noised curves returning all three parameters correctly, is used to evaluate the performance of individual sampling pattern. Matching error is then (1.0 – a), whose natural log value is generally negative.
Results
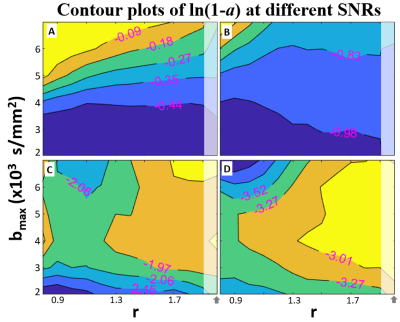
Figure 3 shows contour plots of ln(1.0 – a) for simulations with different bmax (from 2,000 to 7,000) values and different b-spacing power (r) values at four different single channel (real or imaginary) SNR (at b = 0) values: 50 (A), 100 (B), 200 (C), 300 (D). ln(1.0 – a) contours are used to mimic the common appearance of error surfaces, where the lowest [i.e., most negative (bluer)] value represents preferred outcome. For the healthy pancreatic tail, bmax under 3,000 s/mm2 is sufficient for practical achievable SNR (~50-100) and b-spacing pattern is often not critical for these SNRs. Only when SNR ≥ 300, does it become possible to see the benefit of high bmax (D). Overall, the even-spaced b-value array (r = 1) remains a good option.Discussion
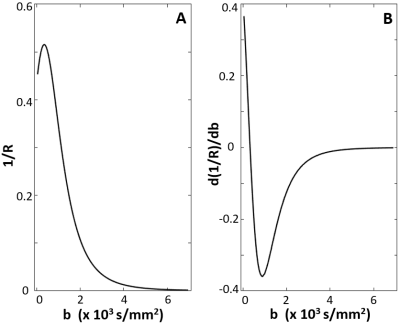
Under a constant acquisition-time constraint, the optimal DWI data acquisition strategy for the pancreatic tail is shown to be an even-spaced b-array with a maximum b-value under ~3,000 s/mm2. With an up-sampled b-array, Figure 4 provides some additional insights. In 4A, the numerically determined radius (R) of curvature reciprocal for the “true” DWI curve (Fig. 1 solid curve) is plotted against the b‑values. When 1/R approaches zero, the b-space decay approaches straight line. Fig. 4B plots the derivative of (1/R) with respect to b-values. This quantifies how quickly the curvature changes. It is obvious the most dramatic changes occur when b < 3,000 s/mm2 for normal pancreatic tail, matching the Fig. 3 simulation results. It is worth noting that the current optimized strategy should work for pancreatic head and body as well, as observed diffusivity is similar in the entire pancreas.7 It is expected that different tissue types with different DWI decay patterns will have different optimal strategies. For example, a prostate lesion has a significantly lower diffusivity, and the Fig. 4B analogy remains significantly non-zero for b-values over 4,000 s/mm2. For this, an r > 1 and bmax > 4,000 can be a preferred strategy (data not shown).Acknowledgements
Grant Support: Brenden-Colsen Center for Pancreatic Care.
Thorsten Feiweier (Siemens) for providing the work-in-progress sequence for DWI data acquisition.
References
1. Baker, Moloney, Li, Gilbert, Springer, PISMRM, 28:3612 (2019).
2. Baker, Moloney, Li, Wilson, Barbara, Maki, Gilbert, Springer, PISMRM, 28:3585 (2019).
3. Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E. Neuroimage. 142:394-406 (2016).
4. Does, Olesen, Harkins, Serradas-Duarte, Gochberg, Jespersen, Shemesh. Magn Reson Med. 81:3503-3514 (2019).
5. Reci, Ainte, Sederman, Mantle, Gladden, MRI, 56:14-18 (2019).
6. Cardenas-Blanco, Tejos, Irarrazaval, Cameron. Concepts Magn Reson Part A 32A:409 – 416 (2008).
7. Barral, Taouli, Guiu, Koh, Luciani, Manfredi, Vilgrain, Hoeffel, Kanematsu, Soyer. Radiology. 274:45-63 (2015).
Figures