3639
Oscillating Gradient Spin Echo-Based Time-dependent Diffusivity Reflects Regional Microstructure Differences in Human White Matter1GE Global Research, Niskayuna, NY, United States, 2Uniformed Services University of the Health Sciences, Bethesda, MD, United States, 3Walter Reed National Military Medical Center, Bethesda, MD, United States, 4GE Healthcare, Stockholm, Sweden
Synopsis
Diffusivity measurements of the human brain have been shown to increase at short diffusion time by using oscillating gradient spin echo (OGSE). The time-dependent diffusivity averaged in large white matter parcels has been measured to assess microstructure characteristics. In this study, we assessed time-dependent diffusivity in finer white matter parcels to study the regional microstructure differences. In four healthy volunteers, we consistently observed higher parallel diffusivity values and higher increasing rate over OGSE frequencies in two sub-parcels of the genu and two of the splenium in the corpus callosum, compared to other regions, indicating regional microstructure differences of the brain.
INTRODUCTION
The parallel diffusivity (PD) measured along the axonal fiber directions has been demonstrated to vary with different diffusion times1,2, reflecting the randomly distributed restrictions along the fibers. Time-dependent PD measurements have been applied to estimate the correlation length and the tortuosity limit in human brain3, which may provide a way to assess axonal beading and swelling in disease2,4. Oscillating gradient spin echo (OGSE) achieves a short diffusion time and is ideal for assessing the 3~6 µm length scale along the axon fiber direction in human brain5. Current PD measurements were averaged in relatively large white matter parcels and may not detect subtler regional differences. In this study, we proposed to assess the regional microstructure differences in finer white matter parcels, first in the corpus callosum, via OGSE-based time-dependent PD measurements using a high-performance gradient MRI (MAGNUS) in healthy human subjects.METHODS
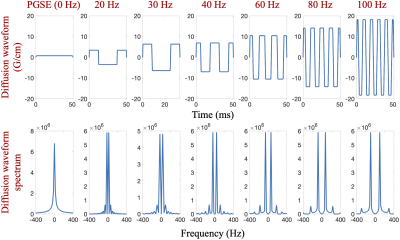
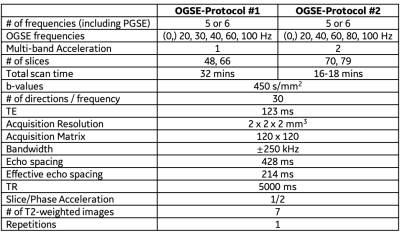
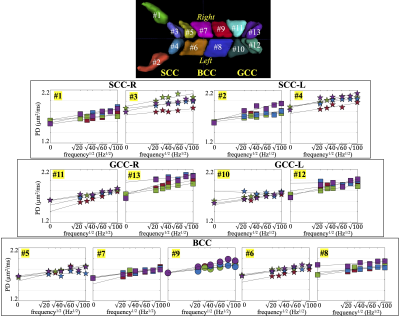
MRI Acquisition: In this IRB-approved study, four healthy volunteers were scanned in a 3.0T MRI system (MR750, GE Healthcare, WI) modified with a head-only MAGNUS gradient coil achieving 200 mT/m amplitude and 500 T/m/s slew rate for each gradient axis6. A 32-channel phase-array head coil (Nova Medical, MA) was used. OGSE cosine-modulated trapezoid waveforms with different frequencies5 were used for diffusion encoding as shown in Figure 1. Additionally, a pulsed gradient spin echo (PGSE) diffusion acquisition with long waveform duration7 was also obtained in two subjects, to sample the 0 Hz in the diffusion spectrum. Imaging parameters were listed in Table 1. T1-weighted MPRAGE anatomical structural images were also acquired.Image reconstruction: All OGSE and PGSE images were distortion corrected and registered to T1-weighted images. Mean/Parallel/Radial diffusivities and fractional anisotropy (FA) were calculated for each frequency. White matter parcels were segmented and registered to a custom brain atlas with 72 white matter parcels8, using the FA map. The parcels of the corpus callosum are shown in Figure 2. Mean values of PD were calculated over all pixels in each white parcel.
Data analysis: The mean PD of all frequencies at each TE was fitted to the 1-dimensional short-range disorder model2,3, PD(f) = D0 + A・f1/2, where the intercept D0 is an measurement of tortuosity, and the slope A correlates with the correlation length of randomly distributed restrictions along the fiber direction.
RESULTS
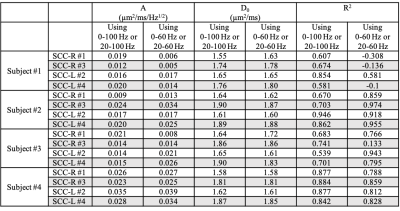
PD measurements of the corpus callosum are shown in Figure 2. The left (L) and right (R) regions of the genu (GCC), body (BCC) and splenium (SCC) with sub-parcels in each region are separately analyzed. Brain left-right symmetric sub-parcels are represented with same markers. PD measurements of all parcels increase as OGSE frequency increases. In the genu and splenium, four sub-parcels (#3 and #4 in SCC, #13 and #12 in GCC) have consistently higher PD measurements than the other parcels in the corpus callosum. And these parcels are anatomically brain left-right symmetric. Additionally, the PD measurements of each left-right sub-parcel pair are in similar ranges, e.g., #3 in SCC-R and #4 in SCC-L. The PD measurements of BCC are shown to have less differences across sub-parcels.In two left-right symmetric splenium sub-parcels, #3 in SCC-L and #4 in SCC-R, the increase of PD at high frequencies (80-100 Hz) appear to be less than the increase of PD at low frequencies (0-60 Hz). The intercepts D0 and slopes A of the splenium sub-parcels in the 1-dimensional short-range disorder model obtained from two fitting using all frequencies and only low frequencies, respectively, are listed in Table 2. In Subjects #2-#4, the slope A estimated using only low frequencies is higher compared to the slope estimated using all frequencies, and the intercept D0 is lower.
DISCUSSION AND CONCLUSION
In this work, we have analyzed regional time-dependent parallel diffusivity in the carpus callosum of healthy volunteers using OGSE diffusion imaging on MAGNUS 3.0T MRI scanner. The cosine-modulated OGSE waveform with 100 Hz frequency and b-value of 450 s/mm2 is enabled by the high amplitude and slew rate of MAGNUS, compared to whole-body MRI systems. PD increase at high OGSE frequencies was observed, which is consistent with previous studies5. The range of PD values in the genu and splenium varies across different sub-parcels, indicating regional microstructure differences in the corpus callosum. Therefore, the time-dependent diffusivity of the white matter is needed to be analyzed regionally, rather than averaged over large parcels. Additionally, PD measurements of the brain left-right symmetric parcels have similar ranges, indicating similar microstructures in left and right healthy brain.In the preliminary observation, the change of PD increasing rate at high OGSE frequencies in the two splenium parcels suggests that the measured diffusion length scale may be approaching the characteristic length of the microstructures. It indicates a potential change of diffusion measurements from the long-time diffusion regime to the inter-medium/short-time diffusion regime at this cross-over frequency. The measurement of the cross-over frequency is important to reliably determine the correlation length that was discussed in previous studies1. However, higher OGSE frequencies above 100 Hz need to be obtained to further analyze the potential change. Future studies will also include correlating metrics from time-dependent diffusivities with tractography and NODDI9 to analyze the potential biophysics of the observed regional microstructure differences.
Acknowledgements
The authors acknowledge the funding support from U.S. Department of Defense, W81XWH-16-2-0054. The opinions or assertions contained herein are the views of the authors and are not to be construed as the views of the U.S. Department of Defense, Walter Reed National Military Medical Center, or the Uniformed Services University. The authors thank Eric Fiveland, Joseph Piel, and Keith Park for the system integration, and Gail Kohls and Kimberly Greenfield for subject recruitment and performing clinical MRI scans.References
1. Fieremans E, Burcaw LM, Lee H-H, Lemberskiy G, Veraart J, Novikov DS. In vivo observation and biophysical interpretation of time-dependent diffusion in human white matter. NeuroImage 2016;129:414-427.
2. Lee H-H, Papaioannou A, Kim S-L, Novikov DS, Fieremans E. A time-dependent diffusion MRI signature of axon caliber variations and beading. Communications Biology 2020;3(1):354.
3. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences 2014;111(14):5088-5093.
4. Burcaw LM, Fieremans E, Novikov DS. Mesoscopic structure of neuronal tracts from time-dependent diffusion. NeuroImage 2015;114:18-37.
5. Tan ET, Shih RY, Mitra J, Sprenger T, Hua Y, Bhushan C, Bernstein MA, McNab JA, DeMarco JK, Ho VB, Foo TKF. Oscillating diffusion-encoding with a high gradient-amplitude and high slew-rate head-only gradient for human brain imaging. Magnetic Resonance in Medicine 2020;n/a(n/a).
6. Foo TKF, Tan ET, Vermilyea ME, Hua Y, Fiveland EW, Piel JE, Park K, Ricci J, Thompson PS, Graziani D, Conte G, Kagan A, Bai Y, Vasil C, Tarasek M, Yeo DTB, Snell F, Lee D, Dean A, DeMarco JK, Shih RY, Hood MN, Chae H, Ho VB. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magnetic Resonance in Medicine 2020;n/a(n/a).
7. Arbabi A, Kai J, Khan AR, Baron CA. Diffusion dispersion imaging: Mapping oscillating gradient spin-echo frequency dependence in the human brain. Magnetic Resonance in Medicine 2019;n/a(n/a).
8. Li X, Bhushan C, Singanamalli A, Tan ET, Sperl JI, Niogi S, Tsiouris J, Mukherjee P, Masdeu J, Shetty T, Marinelli L. Single-subject Voxel-based Analysis for mTBI using Multi-shell Diffusion MRI. 2017. p 3266.
9. Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. NODDI: Practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage 2012;61(4):1000-1016.
Figures