3629
3D Whole Heart Grey-blood PSIR Slow Infusion Imaging for High-resolution Isotropic LGE Imaging1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 2Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 3MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
Synopsis
A free-breathing water/fat motion corrected 3D grey blood phase sensitive inversion recovery (PSIR) late gadolinium enhancement (LGE) technique that achieves whole heart coverage and provided excellent agreement with 2D grey blood LGE MRI has been recently proposed. However, contrast washout due to the relatively long scan time of ~10 minutes may impact scar detection and limit spatial resolution. We therefore sought to investigate the feasibility of high-resolution slow infusion motion-corrected 3D grey blood PSIR-LGE in comparison with a conventional clinically used breath-held 2D grey blood PSIR-LGE MRI technique. Here we report first qualitative and quantitative results.
Introduction
A free-breathing water/fat motion corrected 3D grey blood phase sensitive inversion recovery (PSIR) late gadolinium enhancement (LGE) technique that achieves whole heart coverage and provides excellent agreement with 2D grey blood LGE MRI has been recently proposed1. This approach employs Dixon encoding to provide complimentary fat images. However, contrast washout due to the relatively long scan time of ~10 minutes may impact scar detection2 and limit the spatial resolution3. Moreover, this approach only corrected for 2D translational motion, which may be insufficient in patients with highly irregular breathing patterns and at higher image resolution. To address the above limitations, we sought to investigate the feasibility of slow infusion 3D grey blood LGE MRI in combination with 3D undersampled non-rigid motion corrected reconstruction4. Images were acquired after slow infusion injection of 0.15 mmol/kg GADOVIST contrast agent at 0.3 ml/s5. The proposed grey-blood 3D LGE protocol was evaluated in 7 patients with suspected cardiovascular disease and compared against a clinically used 2D breath hold grey-blood PSIR LGE protocol. Here we report first qualitative and quantitative results with respect to image quality.Methods
Acquisition & Reconstruction: 2D grey-blood PSIR images were acquired ~6 minutes after slow infusion injection of 0.15 mmol/kg GADOVIST contrast agent at 0.3 ml/s, while 3D grey-blood PSIR images were acquired ~12 minutes after slow infusion injection using the framework described in Figure 1. Two interleaved gradient echo volumes with 3 fold undersampling and two-point Dixon encoding6 were acquired using an undersampled variable-density Cartesian trajectory7. Image navigators (iNAVs)8 are integrated in the sequence to enable 100% respiratory scan efficiency and predictable scan time. The first volume is acquired with an IR pulse preceding the imaging sequence and the inversion time is chosen to null the blood signal on the magnitude reconstructed images. The second volume is acquired with no preparation pulse and small flip angle. Beat-to-beat 2D translational motion correction (foot-head and left-right) is applied to all four echoes and after respiratory binning, with the FH motion estimated from iNAV, all four echoes are reconstructed with non-rigid motion corrected iterative SENSE9 reconstruction. To separate both volumes into water/fat images, a water/fat algorithm with magnitude based B0 estimation and phase unwrapping (B0-NICEbd)10 is used. To store the signal polarity for each voxel, an intermediate PSIR reconstruction between in-phase echoes of both acquired volumes is performed. That polarity is then reapplied to the IR prepared water volume to generate the water PSIR images.Imaging & Analysis: Seven patients (6 males, 57±14 years-old) with suspected cardiovascular disease were imaged with the proposed high-resolution 3D grey-blood PSIR slow infusion approach on a 1.5T scanner (Siemens Magneton Aera). Imaging parameters included: coronal orientation, FOV=320x320x104-128mm3, 1.5mm3 isotropic resolution, bandwidth=600Hz/px, TR/TE1/TE2=6.41/2.38/4.76ms, FA=25° and 5° for IR prepared and non-prepared volumes, 14 echoes for iNAV acquisition with FA=3°, acquisition time = 9.5±2.6min. 3D grey-blood PSIR images were compared to the breath-held 2D grey-blood PSIR images both acquired after slow infusion. Image quality assessment was performed using a 4-point Likert scale (1: non-diagnostic, 4: excellent diagnostic quality).
Results
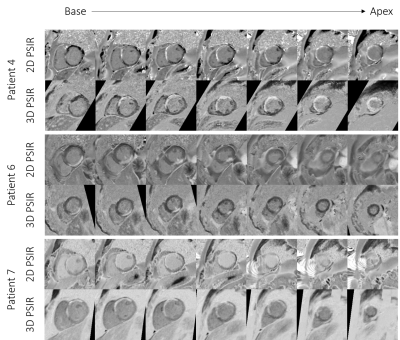
Due to the isotropic resolution of the grey-blood 3D PSIR LGE acquisition, images could be reformatted in short-axis, 2-chamber and 4-chamber orientations as seen on Figure 2 and Figure 3 for two representative patients. Comparison between the grey-blood 2D PSIR sequence and the proposed grey-blood 3D PSIR approach can be seen in Figure 4 in short axis for 3 patients. Comparable image quality can be observed between the 2D PSIR LGE and 3D PSIR LGE images (Figure 5). Slightly lower image quality score can be observed in patients P2, P3 and P6 with 3D PSIR LGE due to remaining water/fat swaps (P2) and motion artifacts (P3 and P6). Improved image quality was observed in patients P5 and P7. Motion artifacts can be seen on the apical short axis in P7 (Figure 4), which may obscure the scar. That could be due to breath-holds, which are eliminated with the proposed free-breathing technique.Conclusion
The proposed high-resolution 3D grey-blood PSIR slow infusion protocol has been successfully tested in seven patients enabling scar visualisation in water PSIR volume while also providing high-resolution fat images. We observed good agreement between the conventional 2D grey-blood PSIR image and proposed 3D grey-blood slow infusion high-resolution PSIR images. Image quality was comparable between the 2D PSIR LGE and 3D PSIR LGE images. Future work will include investigating this protocol in a larger cohort of patients and in comparison with bolus injection.Acknowledgements
This work was supported by EPSRC (EP/L015226/1, EP/P032311/1, EP/P007619/1 and EP/P001009/1) and the Wellcome/EPSRC Centre for Medical Engineering (NS/A000049/1).
References
1. Milotta, G. et al. 3D Whole-heart Motion Compensated Grey-blood Late Gadolinium Enhancement Imaging. ISMRM 2020, Abstract 2045.
2. Munoz, C. et al. Motion-corrected 3D whole-heart water-fat high-resolution late gadolinium enhancement cardiovascular magnetic resonance imaging. J. Cardiovasc. Magn. Reson. 22, 53 (2020).
3. Bi, X., Carr, J. C. & Li, D. Whole-heart coronary magnetic resonance angiography at 3 Tesla in 5 minutes with slow infusion of Gd-BOPTA, a high-relaxivity clinical contrast agent. Magn. Reson. Med. 58, 1–7 (2007).
4. Cruz, G., Atkinson, D., Henningsson, M., Botnar, R. M. & Prieto, C. Highly efficient nonrigid motion-corrected 3D whole-heart coronary vessel wall imaging. Magn. Reson. Med. 77, 1894–1908 (2017).
5. Tandon, A. et al. A clinical combined gadobutrol bolus and slow infusion protocol enabling angiography, inversion recovery whole heart, and late gadolinium enhancement imaging in a single study. J. Cardiovasc. Magn. Reson. 18, 1–7 (2016).
6. Foley, J. R. J. et al. Feasibility study of a single breath-hold, 3D mDIXON pulse sequence for late gadolinium enhancement imaging of ischemic scar. J. Magn. Reson. Imaging 49, 1437–1445 (2019).
7. Prieto, C. et al. Highly efficient respiratory motion compensated free-breathing coronary mra using golden-step Cartesian acquisition. J. Magn. Reson. Imaging 41, 738–746 (2015).
8. Henningsson, M. et al. Whole-heart coronary MR angiography with 2D self-navigated image reconstruction. Magn. Reson. Med. 67, 437–445 (2012).
9. Pruessmann, K. P., Weiger, M., Börnert, P. & Boesiger, P. Advances in sensitivity encoding with arbitrary k -space trajectories. Magn. Reson. Med. 46, 638–651 (2001).
10. Liu, J., Peters, D. C. & Drangova, M. Method of B0 mapping with magnitude-based correction for bipolar two-point Dixon cardiac MRI. Magn. Reson. Med. 78, 1862–1869 (2017).
Figures