3626
Evaluating the Myocardial Diffusion Status in Cardiac Amyloidosis: A Novel Intravoxel Incoherent Motion Diffusion-weighted MR Imaging Study
Mengdi Jiang1, Xianghua Huang2, Guifen Yang3, Weiqiang Dou4, and Yong Shen5
1Department of Medical Imaging, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 2National Clinical Research Center of Kidney Disease, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 3Department of Nuclear Medical, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 4GE Healthcare,MR Research China, BeiJing, China, 5GE Healthcare,MR Enhanced Application China, BeiJing, China
1Department of Medical Imaging, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 2National Clinical Research Center of Kidney Disease, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 3Department of Nuclear Medical, Jinling Hospital, Medical School of Nanjing University, NanJing, China, 4GE Healthcare,MR Research China, BeiJing, China, 5GE Healthcare,MR Enhanced Application China, BeiJing, China
Synopsis
The purpose of this study was to evaluate the diagnostic value of myocardial diffusion and mechanical properties by using IVIM-DWI imaging, feature tracking and native T1 in patients with cardiac amyloidosis (CA). The relationships of strain, native T1, IVIM-derived parameters (ADCslow, ADCfast and F) and late gadolinium enhancement (LGE) were analyzed based on six mid-ventricle subregions. Significantly different IVIM related parameters were found in patients than healthy controls. Furthermore, IVIM parameters also showed significant correlation with peak strain and nativeT1. With these findings, IVIM parameters has proven as effective biomarkers in the diagnosis of cardiac microcirculation in CA patients.
Introduction
Cardiac amyloidosis (CA) is the condition in which amyloid fibrils occur in the extracellular space of heart, characterized by the aggregation and deposition of misfolded protein1. Cardiac magnetic resonance imaging has increasingly been a central role in non-invasive diagnosis of CA due to the provided tissue characterization, including evaluation of native T1 signal, assessment of late gadolinium enhancement (LGE), extracellular volume (ECV) and feature tracking measurement2.Recently, diffusion weighted imaging (DWI) using multiple b values, permitting imaging of intravoxel incoherent motion (IVIM) in tissues, can provide microstructural details with respect to water molecular motion between myocytes, microcirculation and vascular flow3,4. At present, the validation and feasibility of IVIM for assessing acute myocardial infarction and hypertrophic cardiomyopathy has been proven previously5,6, while leaving a gap in other diseases.
In this study, we hypothesized that CA patients, relative to healthy controls, would exhibit different diffusion behaviors using IVIM, because of the fibrils deposition,and also postulated that IVIM parameters would correlate with the feature tracking and native T1 relaxation time, reflecting amyloid burden and thus being reliable biomarkers in the assessment of CA.
Materials and Methods
This clinical study was approved by the local Institution Review Board. Written informed consent was obtained from all subjects.Subjects
9 patients (54±5 years, 6 male) and 21 healthy (52±11 years, 14 male) were prospectively recruited in this study. All patients have histologically proven amyloidosis (positive Congo red staining of abdominal fat, kidney or bone marrow biopsies) and echocardiographic findings or biomarker typical for cardiac involvement.
MRI experiment
Cardiac Magnetic Resonance imaging (CMR) was performed on a 3.0-Tesla scanner (GE750, Milwaukee, WI, USA) with 8-channel cardiac coil employed. Cine imaging, pre/post T1 mapping, DWI-IVIM (b-values 0, 50, 100, 200, 400, and 600s/mm2), and LGE imaging were applied for heart imaging in axial view. Detailed scan parameters were shown in Table 1.
Data analysis
One senior cardiovascular radiologist was employed to measure all IVIM images twice within a 1-month interval. The intra-observer agreement was test using both sets of measurements. The mean results were further obtained and used for data analysis. Additionally, to test inter-observer agreement, one measurement of randomly chosen data from 6 subjects was also acquired from another radiologist with relevant expertise. Using bi-exponential model7, IVIM derived parameters of true diffusion ADCslow, pseudo diffusion ADCfast and perfusion fraction F were obtained accordingly. Other data analyses, including feature tracking and native T1 mapping, were performed using cvi42 (Circle Cardiovascular Imaging, Calgary, Canada) by the employed senior radiologist. The region of interest in all MR data was limited to the mid-ventricle area and separated into 6 segments. All patients were subdivided into LGE(+) and LGE(−) groups based on the imaging enhancement on LGE images.
Statistical analysis
All statistical analyses were performed in SPSS software (SPSS V23.0, IBM SPSS Inc). Interobserver and intra-observer reliability of the IVIM image parameters was assessed by using intraclass correlation coefficient (ICC) analysis. Unpaired two-tailed t-test was applied to compare IVIM associated parameters in each mid-ventricle segment between healthy and patients. Pearson’s correlation analysis was employed to evaluate the relationship between IVIM image parameters and peak strain or native T1. P value < 0.05 was considered the significant threshold.
Results
In total, 153 segments from patients and healthy controls were acquired with complete IVIM associated parameters, strain and native T1. Fig.1 showed IVIM images from short-axis view. 9 CA patients were further divided into 24 LGE(+) and 19 LGE(−) segments.Intra- and inter-observer agreement of all IVIM parameters measurement were 0.93, 0.84 for ADCslow, 0.92, 0.87 for ADCfast and 0.94, 0.91 for F, respectively.
Compared with healthy controls, CA patients showed significantly higher ADCslow (2.50±0.81×10-3mm2/s vs 2.17± 0.74 ×10-3mm2/s, p=0.017), while comparable ADCfast (0.18±0.08mm2/s vs 0.20±0.08mm2/s, p=0.084) and F (0.45±0.12 vs 0.43±012, p= 0.277) were observed between CA and healthy segments.
In additionally, LGE(−) segments exhibited an increased ADCslow (2.55±0.78×10-3mm2/s) than normal segments (p=0.044)(Fig. 2A). LGE(+) segments exhibited a decreased ADCfast (0.16±0.08mm2/s) than healthy segments (p=0.02)(Fig. 2B). Using Pearson correlation analysis, only ADCfast in LGE(+) CA group showed significant correlation with peak strain or native T1, respectively (all p<0.05; Fig. 3). A representative case of a healthy control and a CA patient is displayed in Fig. 4.
Discussion
This study investigated the clinical value of cardiac DWI-IVIM in the evaluation of CA patients and assessed the feasibility of IVIM for detecting hypoperfusion caused by amyloid deposition. IVIM derived parameters of ADCslow and ADCfast were found to be significantly altered in CA patients relative to healthy controls. Especially, amyloid burden severity could be quantified using ADCfast, showing significant relationship with peak strain and T1 values of CA patients. Future research may identify amyloidosis specific patterns of IVIM parameters, which might be an advantage over rather unspecific parameters such as native T1 that shows elevated value in multiple cardiac diseases.Conclusion
In conclusion, Cardiac DWI-IVIM has demonstrated the clinical value in the investigation of cardiac microcirculation. The derived ADCslow and ADCfast parameters may have potential to assess the perfusion status in amyloidosis regions for CA patients.Acknowledgements
No acknowledgement found.References
1. Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641-2654. 2. S Dorbala, Y Ando, S Bokhari, et al. ASNC/AHA/ASE/EANM/HFSA/ISA/SCMR/SNMMI expert consensus recommendations for multimodality imaging in cardiac amyloidosis: Part 1 of 2-evidence base and standardized methods of imaging. J Nucl Cardiol, 2019;26(6):2065-2123. 3. Nielles-Vallespin S, Scott A, Ferreira P, Khalique Z, Pennell D, Firmin D. Cardiac Diffusion: Technique and Practical Applications. J Magn Reson Imaging. 2020;52(2):348-368. 4. Delattre BM, Viallon M, Wei H, et al. In vivo cardiac diffusion-weighted magnetic resonance imaging: quantification of normal perfusion and diffusion coefficients with intravoxel incoherent motion imaging. Invest Radiol. 2012;47(11):662-670. 5. Wu R, An DA, Shi RY, et al. The feasibility and diagnostic value of intravoxel incoherent motion diffusion-weighted imaging in the assessment of myocardial fibrosis in hypertrophic cardiomyopathy patients. Eur J Radiol. doi: 10.1016/j.ejrad.2020.109333. 6. Mou A, Zhang C, Li M, et al. Evaluation of myocardial microcirculation using intravoxel incoherent motion imaging. J Magn Reson Imaging. 2017;46(6):1818-1828. 7. Zhang SX, Jia QJ, Zhang ZP, et al. Intravoxel incoherent motion MRI: emerging pplications for nasopharyngeal carcinoma at the primary site. Eur Radiol. 2014;24(8):1998-2004.Figures
Table 1 Scan parameters for CMR imaging
techniques used. ms=millisecond, mm=millimeter, s=seconds, n/a=not available.
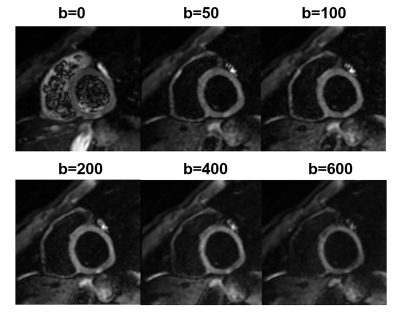
Figure 1 IVIM images
from short-axis view. Representative IVIM images (a volunteer, male,
64 years old) with b values of 0, 50, 100, 200, 400, and 600 s/mm2 showing
the cardiac base of left ventricle at short-axis view.
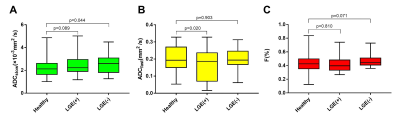
Figure
2 Box plot indicating the distribution of the ADCslow, ADCfast,
and F values among the healthy controls and LGE(+) or LGE(-) patients. LGE= late gadolinium enhancement.
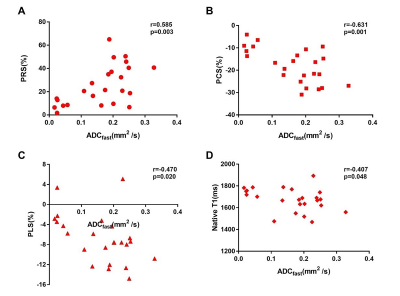
Figure 3 Correlation
analysis between ADCfast with peak radial strain, peak
circumferential strain, peak longitudinal strain and native T1. PRS=peak radial strain, PCS=peak circumferential
strain, PLS=peak longitudinal strain, other abbreviations as in Figure 2.
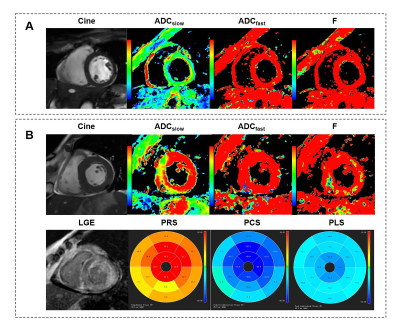
Figure 4 Typical
example of IVIM-derived parameters, strain and LGE for (A) a healthy control
and (B) a LGE(+) patient with cardiac amyloidosis. Other abbreviations
as in Figure 2&3.