3625
Increased SNR and improved reproducibility for cardiac 31P MRS at 7T using compartmentalized spectroscopy1Oxford Centre for Clinical Cardiac Magnetic Resonance Research, University of Oxford, Oxford, United Kingdom, 2Department of Physiology, Anatomy & Genetics, University of Oxford, Oxford, United Kingdom, 3Department of Physics, University of Oxford, Oxford, United Kingdom, 4The Division of MR Research, Johns Hopkins Medicine, Baltimore, MD, United States, 5Wolfson Brain Imaging Centre, University of Cambidge, Cambridge, United Kingdom, 6Department of Imaging Methods, Institute of Measurement Science, Slovak Academy of Sciences, Bratislava, Slovakia
Synopsis
The intrinsically low SNR and long acquisition times of 31P spectroscopy make translation to clinical practice very challenging, even at ultra-high fields. In this study, 12 healthy volunteers were scanned twice at 7T with a short 31P CSI and reduced k-space acquisitions for reconstruction with the SLAM and SLIM algorithms. PCr/ATP ratio and PCr SNR were computed for each scan and coefficients of reproducibility and variability were calculated. Compared to a Fourier based reconstruction of the short 31P acquisition, SNR was significantly improved and PCr/ATP was maintained when SLAM and SLIM reconstructions were used.
Introduction
MRS of high energy phosphorus metabolites provides a unique insight into cardiac metabolism. Of particular interest is the marked difference in PCr/ATP metabolite ratio between the healthy and diseased heart [1] which has been shown to be a significant predictor of cardiac mortality [2]. However 31P MRSI is fundamentally limited by poor SNR, leading to long scan times and poor spectral or low spatial resolution.To reduce the number of phase encodes required for 31P-MRS several compartmentalized spectroscopy techniques, including SLIM[3], SLAM[4], SLOOP[5] etc, have been proposed. These techniques seek to improve upon conventional CSI by incorporating prior knowledge of the anatomical structure of the subject into the reconstruction of spectra and/or the optimization of phase encode gradients.
The fundamental assumption underlying these techniques is that the sample can be divided up into n homogeneous compartments[3], each of which has uniform MR properties, although the exact implementation of SLIM/SLOOP and SLAM are markedly different. Using this knowledge a series of simultaneous equations are constructed:
$$P=GC$$
Where $$$P$$$ is a vector of m phase encoded signals, $$$G$$$ a phase encode operator with dimensions m x n and $$$C$$$ a vector of n separated signals. The equation is then solved by multiplying $$$P$$$ by the inverse of $$$G$$$ to give signals for each compartment.
By matching the spatial response function (SRF) to the compartment of interest, a lower number of phase encodes can be used to achieve the localization allowing for an increase in SNR or reduction in scan time compared to regular CSI.
Ultra-high fields increase the achievable SNR in 31P-MRS almost 3-fold[6], however, a further increase in SNR could improve precision or reproducibility. At 7T, the B0 and B1 fields are highly in-homogeneous and it is not yet clear if this will prevent the SLAM and SLIM algorithms from effectively separating the spectra for each compartment due to the underlying homogeneity assumption of the techniques. This means that previous work at 1.5 and 3T may not be applicable at 7T.
In this work, we asses the feasibility of using compartmentalized spectroscopy techniques for cardiac 31P MRS at 7T.
Methods
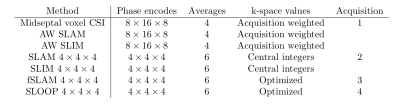
12 healthy volunteers (6M/6F, BMI=23±3 kg/m2, age=29±4 years) were scanned using a Siemens 7T Magnetom system (Erlangen, Germany), equipped with a dual-tuned custom built RF-coil (quadrature Tx/Rx for 31P and 10cm loop for 1H[7]). A short axis localizer, matching the planned CSI acquisition was collected, followed by four 3D 31P CSI acquisitions (detailed in figure 1) to allow each of the proposed reconstruction methods to be analysed. While the non-optimized acquisitions were completed (acquisition 1, 2) the matched short axis localisers were manually segmented (heart, chest wall, other) and optimized fSLAM and SLOOP phase encode coordinates were calculated. All CSI scans were matched to take a 6.5 minutes.After completion of the scan, the spectra were reconstructed using each of the proposed methods and fitted using the OXSA Matlab toolbox AMARES implementation[8] (figure 2). For each fitted spectrum PCr/ATP ratios, PCr SNR and coefficients of reproducibility (for PCr/ATP ratio) were computed. Wilcoxon signed-rank paired tests (α=0.05/comparisons, Bonferroni correction), were used to compare the methods with the analysis of a single midseptal voxel, which is most commonly used for cardiac 31P CSI. A small number of spectra were un-analysable and excluded from further analysis (1 participant with excessive movement + 3% of remaining data-points with very high liver contamination).
Results and Discussion
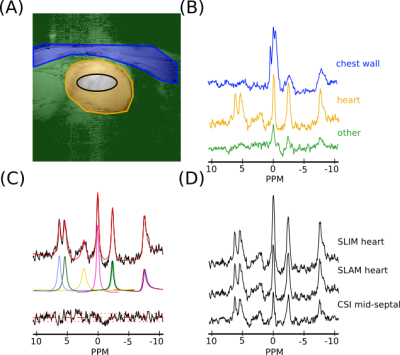
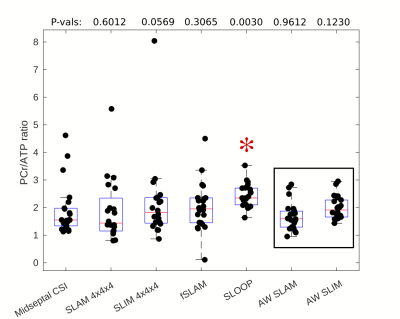
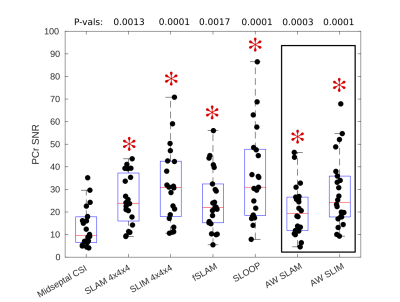
An example compartmentalization of one short axis slice through the chest and the reconstructed spectra for that volunteer is shown in figure 2. As expected the heart has a lower PCr to ATP ratio than the chest wall and the 2,3-DPG resonance is clearly visible in the heart spectra due to blood in the cardiac chambers.Box-plots of PCr/ATP ratio and PCr SNR are shown in figures 3 and 4 respectively. A table summarizing the computed coefficients of reproducibility and variation is shown in figure 5. All compartmentalised spectroscopy techniques had a significantly higher SNR (Wilcoxon signed-rank, paired, α=0.05/6) than simple analysis of the midseptal voxel, in line with previously reported results [4]. The computed PCr/ATP values were within a plausible range of values[6][9], however, the SLOOP value was significantly higher (Wilcoxon signed-rank, paired, α=0.05/6) than the midseptal value.
Unlike the other compartmentalized techniques, AW SLAM and SLIM are a re-analysis of the midseptal voxel CSI acquisition, making implementation simple, however they still achieved lower (better) coefficients of variability and reproducibility than the midseptal value, while maintaining a similar PCr/ATP ratio. Differences in the reproducibility reported here and by Ellis et al.[9] can be attributed to different RF hardware, however the relative improvement seen here suggests that an improvement would be seen with that hardware too.
Conclusion
In this study we show that compartmentalized spectroscopy techniques can achieve a significant increase in SNR and improved inter-scan reproducibility compared to our existing Fourier based midseptal voxel method while maintaining the PCr/ATP ratio. This suggests that the B1 and B0 in-homogeneity present at 7T is not a significant barrier to the use of compartmentalised MRS reconstruction techniques, with the right acquisition.Acknowledgements
This work was supported by funding from the Engineering and Physical Sciences Research Council (EPSRC) and Medical Research Council (MRC) [grant number EP/L016052/1]. DJT is funded by a BHF Senior fellowship [FS/19/18/34252]. JJM would like to acknowledge the support of a Novo Nordisk Postdoctoral Fellowship, and the financal support of St Hugh's and Wadham College. CTR and LV are funded by a Sir Henry Dale Fellowship from the Wellcome Trust [098436/Z/12/B]. Support of the Slovak Grant Agencies VEGA [2/0003/20] and APVV [#19–0032] is also gratefully acknowledged.References
[1] O. J. Rider et al. Int. Journ. Card. Imag. 29:1043-1050, 2013
[2] S. Neubauer et al. Circ. 96:2190-2196, 1997
[3] X. Hu et al, Magn. Reson. Med. 8, 314–322, 1988
[4] Y. Zhang et al. J. Magn. Reson. 218, 66-76, 2012
[5] M. Kienlin et al. J. Magn. Reson. 94, 268-287,1991
[6] C. T. Rodgers et al. Magn. Reson. Med. 72, 304-315, 2014
[7] Schaller et al. Proc. Intl. Soc. Magn. Reson. Med. Singapore, 2016
[8] Purvis et al. PLoS ONE. 12(9):e0185356, 2017
[9] J. Ellis et al. NMR in Biomed. 32:e4095, 2019
Figures