3621
Microstructure-Based Simulation of Myocardial Diffusion Using Extended Volume Confocal Microscopy1Radiology, Stanford University, Palo Alto, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Auckland Bioengineering Institute, University of Auckland, Auckland, New Zealand
Synopsis
Aiming to improve noninvasive assessment of tissue microstructure, a physics-based simulation of diffusion tensor imaging (DTI) was used to compare helix angles between DTI and structure tensor (ST) analyses of a confocal myocardial image stack. Rodent myocardium was imaged using extended volume confocal microscopy producing a high-resolution image volume, which was segmented into intracellular and extracellular compartments. The image volume was divided into voxel-blocks, which served as the DTI simulation voxels. The DTI acquisition showed good agreement with both the ST and manually measured helix angles. This proof-of-concept work demonstrates the feasibility of direct comparison of confocal images with DTI.
Introduction
Myocardial biopsy and histology are the current reference standard to diagnose conditions that afflict the microstructure of the myocardium, such as myocarditis and transplant rejection. These invasive procedures have an associated risk of damage to the heart and lungs. Noninvasive measurement of cardiac microstructure would avoid these risks. Diffusion tensor MRI probes the Brownian motion of water molecules to gain information about micro-structures within the imaging voxel. Diffusion weighted acquisitions are performed along different directions, and the diffusion tensor model can be fit to the image data. Cardiac diffusion tensor imaging (cDTI) is a technique for studying cardiac microstructure, for which the primary eigenvector aligns with the myofiber direction (Refs 5-7). Methods such as tissue clearing and extended volume confocal microscopy allow for image acquisition of millimeter-scale volumes with sub-micron spatial resolution. High resolution confocal images are amenable to structure tensor (ST) analysis, which can also be used to assess myofiber direction (Ref 4). Herein we applied an existing physics-based diffusion simulation framework (Ref 1) to a myocardial image volume and compared the results against the image-based ST analysis. We demonstrate feasibility of direct comparison between confocal images of myocardium with cDTI.Methods
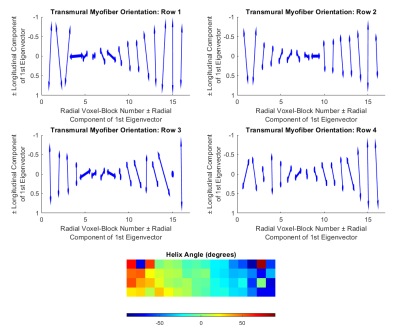
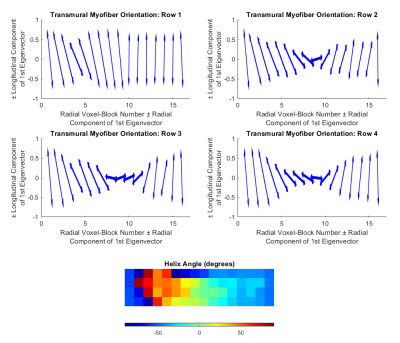
Figure 1 provides an overview of the study, and shows histology and segmented images. A rodent heart was excised and stained with picrosirius red. A transmural block of the left ventricular free wall was cut, dehydrated, embedded in resin, and mounted on a precision stage. Extended-volume confocal microscopy (Ref 2) produced the image volume (3952×988×247 microns, at 1 micron voxel size). The ilastik (v 1.3.3, Ref 3) pixel classification software was used to segment the image volume into myocyte, extracellular matrix and blood vessel. The segmented image was then divided into 247×247×247 micron voxel-blocks, producing 64 voxel-blocks (Figure 2). For each voxel-block segmented, an intra/extra-cellular mask was generated to perform particle-based and MR diffusion simulation (Ref 1). A total of 1000 molecules per voxel-block were simulated with coefficients of diffusion of 3×10-3 mm²/s and 2×10-3 mm²/s, dT=10µs for the extracellular and intracellular compartments respectively with no permeability. The diffusion MR sequence simulated corresponded to a motion-compensated cDTI sequence with a b-value of 1000 s/mm2, 12 diffusion encoding directions and with 4 averages. A diffusion tensor was fit to the signals and the primary, eigenvector was calculated. The ST was estimated for each of the 64 voxel-blocks by calculating the gradient of intensity along each of the confocal image directions (Figure 2), and these derivatives were used to construct the ST. The third eigenvector of the ST has the least change in contrast along its direction, and aligns with the myofiber direction (Ref 4). The helix angle (HA) was obtained by projecting the myofiber orientation onto the circumferential-longitudinal plane, and calculating the angle between that projection and the circumferential direction.Results
The diffusion tensor simulation produced a transmural helix angle of 49° ± 17° at the epicardium, -6° ± 4° at the midwall, and -59° ± 17° at the endocardium. This transition was smoothly varying as it progressed through the voxel-blocks from epicardium to endocardium (Figure 3). The structure tensor analysis produced a helix angle of -55° ± 1° at the epicardium, -13° ± 30° at the midwall, to -48° ± 1° at the endocardium. The expected helix angle trend was observed in three of the voxel-block rows, with the top voxel-block row deviating from this trend (Figure 4). Compared with the manually measured helix angle, both the diffusion tensor and ST approaches over-estimated of the HA (Figure 5). The transmural gradient of the change in HA for both the diffusion tensor and ST approaches matched the manually measured HA.Discussion
We demonstrated that a physics-based simulation of cDTI acquisition was able to produce realistic myocardial HA that aligned with histological images of the myocardium, as well as the feasibility of direct comparison between confocal images and cDTI. At the voxel-block level, the cDTI performed similarly to the structure tensor analysis in most instances. The cDTI simulation had similar performance to the image-based structure tensor analysis. This study demonstrates the feasibility of using physics-based DTI simulation to tune acquisition parameters to maximize sensitivity for a given type of microstructural feature or histology specimen. While this study focused on HA, acquisition strategies could in theory be developed to probe cellular size to investigate myocyte hypertrophy, or the diffusion between blood vessel and extracellular matrix as a means to probe microvascular dysfunction. This framework has the potential to allow pathologists to try different diffusion sequences and parameters needed to extract relevant microstructural information for the pathology specimen of interest. One of the limitations of the segmentation method used for this study, was that the boundaries of individual myocytes were not distinctly defined, which would lead to an over-estimation of the diffusion between the intracellular and extracellular compartments. In future work we aim to further compare real and simulated cDTI acquisitions with both cDTI and histological imaging from the same specimen.Conclusion
Using a physics-based simulation, we were able to demonstrated that cDTI and histology can be directly compared, allowing for future radiology-pathology studies.Acknowledgements
We acknowledge funding from the American Heart Association.References
1. Moulin K, Viallon M, Maforo NG, Mazzoli V, Croisille P, Ennis DB. Sensitivity of Cardiac Diffusion Encoding Waveforms to Myocardial Extracellular Volume: A simulation study. ISMRM 28th Annual Meeting, Paris, France {Virtual Conference}, 2020.
2. Sands, G.B., Gerneke, D.A., Hooks, D.A., Green, C.R., Smaill, B.H. and Legrice, I.J., 2005. Automated imaging of extended tissue volumes using confocal microscopy. Microscopy research and technique, 67(5), pp.227-239.
3. Berg, S., Kutra, D., Kroeger, T., Straehle, C.N., Kausler, B.X., Haubold, C., Schiegg, M., Ales, J., Beier, T., Rudy, M. and Eren, K., 2019. Ilastik: interactive machine learning for (bio) image analysis. Nature Methods, pp.1-7.
4. Bernus, O., Radjenovic, A., Trew, M.L., LeGrice, I.J., Sands, G.B., Magee, D.R., Smaill, B.H. and Gilbert, S.H., 2015. Comparison of diffusion tensor imaging by cardiovascular magnetic resonance and gadolinium enhanced 3D image intensity approaches to investigation of structural anisotropy in explanted rat hearts. Journal of Cardiovascular Magnetic Resonance, 17(1), p.31.
5. Scollan, D.F., Holmes, A., Winslow, R. and Forder, J., 1998. Histological validation of myocardial microstructure obtained from diffusion tensor magnetic resonance imaging. American Journal of Physiology-Heart and Circulatory Physiology, 275(6), pp.H2308-H2318.
6. Holmes, A. Alexander, D. F. Scollan, and Raimond L. Winslow. "Direct histological validation of diffusion tensor MRI in formaldehyde‐fixed myocardium." Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine 44.1 (2000): 157-161.
7. Kung, G.L., Nguyen, T.C., Itoh, A., Skare, S., Ingels Jr, N.B., Miller, D.C. and Ennis, D.B., 2011. The presence of two local myocardial sheet populations confirmed by diffusion tensor MRI and histological validation. Journal of Magnetic Resonance Imaging, 34(5), pp.1080-1091.
Figures