3616
Evaluation of cardiac pre- and post-T1 maps registration for extracellular volume computation1Department of Research & Innovation, Olea Medical, La Ciotat, France
Synopsis
The myocardial ECV involves manual segmentation on pre- and post-injection T1 maps, which is a time-consuming process. An initial registration of the two maps represents a suitable solution to reduce the analyze duration. Here, we propose to compare the ECV obtained after the registration against the manual computed one using a STEMI-patients database. If the difference between the two approaches was significant, the bias remained thin (less than 1% for the remote part and 3% for the infarct part). These results and the speed of the registration (1s per slice) tip the scales in favor of its use
Introduction
The myocardial extracellular volume (ECV) is usually computed in regions extracted from a segmentation of the myocardium (mostly corresponding to the American Heart Association -AHA- subdivision)1. The automatic segmentation of the heart is often missing in clinical software oriented toward cardiac MRI. In consequence, the ECV computation requires a time-consuming manual delimitation of myocardium on the T1 maps pre- and post-injection. Image registration may represent a suitable solution to avoid either completely the segmentation step (the user only needs to provide a point inside the right ventricular -RV), or to segment two maps (the user segments only one map, the post-injection T1 map). We aim here to compare the ECV computation based on a motion correction (Moco) against the manual approach.Methods
A database including 73 STEMI-patients acquired 12 months after the acute phase was used for this study. Five short axis slices were scanned for each patient, and an expert manually delineates the myocardium for 1 to 3 slices in pre- and post-injection acquisitions each time, for a total of 148 labeled slices.Then, the images with the higher inversion time (TI) of both pre- and post-injection modified Look-Locker inversion recovery (MOLLI) sequence were transformed into a representation independent of the underlying image acquisition using a Sobel-based filter. The resulting descriptors were used for the Moco driven using the fast symmetric and diffeomorphic demon2.
The manual ECV of the remote part was derived from the averaged T1 of the myocardium using the whole manually defined mask for the pre-injection case and the remote part of the mask in post-injection case. For the ECV of the infarct part, we used the same pre-T1 with the averaged post-T1 of the infarct region of the post-injection mask. On the other hand, the ECV obtained after registration (ECV Moco) was derived from the average pre- and post-T1 of the remote part of the post-injection mask for the remote ECV and from the infarct part of the same mask for the infarct ECV.
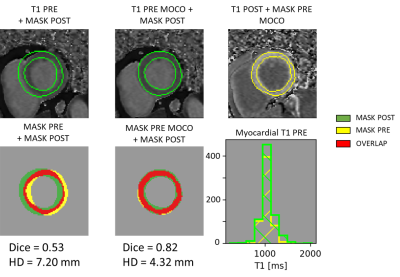
Results analysis compared the ECV against the ECV Moco using a Wilcoxon signed-rank test. To evaluate the effect of the registration on the T1 values of the myocardium, we also computed the divergence between the histograms obtained using the pre- and post-mask respectively on the pre-T1 maps and the registered pre-T1 maps. We used the same range and the same positions of bins for both histograms of myocardial T1 values. The distances used were the L2-normed (L2 div) and the Jeffrey’s divergence (a symmetric Kullback-Leibler divergence)3. An example of the data to analyze is presented in figure 1. To refine the analyzes, we clustered the results in four groups according to their Dice results after registration. The representative Dice value of each group was chosen as its median value.
Results
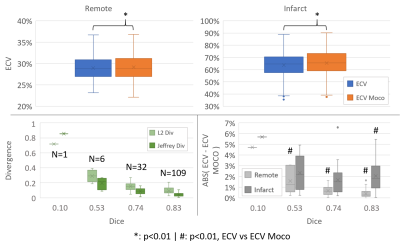
The results are embedded in figure 2. The difference between the manual and registered ECV was significant (p<0.01) for both remote and infarct region (remote: 28.96%+/-2.69% -manual- vs 29.17%+/-2.82% -Moco-; infarct: 63.74%+/-9.78% vs 65.46%+/-9.60%).The study of the divergence revealed a correlation with the Dice: the coefficient of determination (R2) was respectively 0.99, and 0.95 for L2-div and Jeffrey’s divergence. However, for the well enough registered image (Dice of 0.74 and 0.83, representing 141 cases), the median divergence values were less than 0.15 and 0.09 for L2 div and Jeffrey’s divergence, respectively.
We also observed a correlation between the Dice and the median differences between manual and registration based ECV. R2 values were respectively 0.98 and 0.86 for the remote and the infarct region. For the 0.83 Dice group, the median was respectively 0.39% and 2.05%, while the observed maximum difference was respectively 1.62% and 6.61% for the remote and infarct region. The differences were significant (p<0.01) for all the Dice’s group concerning the remote part, but only for the 0.83 Dice group concerning the infarct part.
Discussion & conclusion
As expected, the difference between the estimated ECV obtained by the manual approach and the registration-based approach increases with the decrease of the registration performances. However, more than 95% of the cases were sufficiently registered (Dice>0.6) and even if the registration-induced bias on the ECV values is significant, the ECV absolute difference remained thin (less than 1% for the remote part and 3% for the infarct part). The difference was even thinner when removing registration failure cases. This highlights the need of a registration assessor technique not based on a segmentation of the myocardium.The choice of a demon algorithm for the registration was justified by its fast computation performance (about 1 second per slice on a laptop with 32Go at 2.9GHz). When combining such fast registration strategy with the one-click action to define the RV region, one can expect a quick ECV maps production.
Finally, this study demonstrates the ability of the pre- and post-injected T1 maps registration to significatively reduce the processing time to produce ECV for the operator, thereby superseding the segmentation needs, and stimulating its use in clinical routine.
Acknowledgements
The authors thank Charles de Bourguignon for providing the epi and endocardial contours used for this study.References
1. Messroghli, D. R. et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 19, 75 (2017).
2. Vercauteren, T., Pennec, X., Perchant, A. & Ayache, N. Symmetric Log-Domain Diffeomorphic Registration: A Demons-Based Approach. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2008 (eds. Metaxas, D., Axel, L., Fichtinger, G. & Székely, G.) 754–761 (Springer, 2008). doi:10.1007/978-3-540-85988-8_90.
3. Puzicha, J., Hofmann, T. & Buhmann, J. M. Non-parametric similarity measures for unsupervised texture segmentation and image retrieval. in Proceedings of IEEE Computer Society Conference on Computer Vision and Pattern Recognition 267–272 (1997). doi:10.1109/CVPR.1997.609331.
Figures