3612
Simultaneous Multi-slice Cardiac MR Multitasking for Motion-Resolved, Non-ECG, Free-Breathing Joint T1-T2 Mapping1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 3Siemens Medical Solutions Inc., Los Angeles, CA, United States
Synopsis
Cardiac MR Multitasking has been shown to acquire simultaneous T1 and T2 maps without ECG or breath-holds. Simultaneous multi-slice (SMS) acquisition has the potential to increase the clinical value of CMR by decreasing the exam time. In this work, we developed an SMS, non-ECG and free-breathing MR Multitasking technique and demonstrated that it had consistent T1, T2 measurements when compared with the overall-slower series of reference protocols (MOLLI, T2-prep FLASH).
Introduction
Myocardial T1 and T2 mapping have been used to assess focal and diffuse myocardial pathologies. Current methods typically use a combination of ECG gating and breath-holding, although multi-dimensional methods such as MR Multitasking1 have shown promise for non-ECG and free-breathing T1-T2 mapping. However, Multitasking has so far been demonstrated for 2D single-slice1 imaging in a 1.5 min scan and for 3D2-3 volumetric imaging in 9 min or longer. Clinical protocols typically include T1 and T2 maps in several slices, but not in a full 3D stack, so neither the single-slice nor the 3D volumetric solutions are direct replacements for the conventional series of multiple T1 and T2 mapping scans. Here we combine the MR Multitasking framework with radial simultaneous multi-slice (SMS) acquisition4-7 to perform cardiac-resolved T1/T2 mapping of basal, mid, and apical short-axis slices in a single non-ECG, free-breathing 3-min scan—roughly half the exam time of a conventional six-breath-hold sequence of three T1 slices followed by three T2 slices.Methods
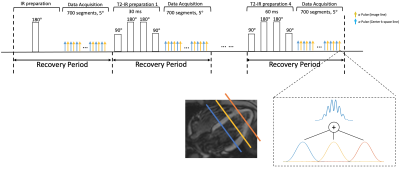
Sequence Design: The proposed continuous 2D sequence uses a radial trajectory modified to collect low-rank tensor auxiliary data interleaved with image data. A tiny golden angle (23.67°) angular increment was used between consecutive imaging readouts. A CAIPIRINHA-type phase modulation pattern was applied to the excitation pulses to achieve a multi-band factor of 3. The auxiliary data is acquired every other readout. Interleaved IR and T2-IR pulses are employed to generate the T1 and T2 contrasts (i.e., Fig. 1). The IR pulse is performed for the first recovery period, whereas the T2-IR pulses with four different preparation times (i.e., 30ms, 40ms, 50ms, 60ms) are performed in the following four recovery periods. The continuous acquisition sequence collects small-angle (i.e., 5°) FLASH readouts after the preparation pulses. Each FLASH excitation pulse was modified to have a different phase modulation so that it can excite 3 slices simultaneously with discrete Fourier slice encoding.Image Reconstruction: The images acquired in the Multitasking framework can be represented as a 5-way tensor $$$ \boldsymbol{\mathcal{A}} $$$ with voxel location index $$$ \mathbf{r}=(x,y,z) $$$, a T1 recovery dimension, a T2 decay dimension, a cardiac motion dimension, a respiratory motion dimension. The high correlation along and across multiple time dimensions induces $$$ \boldsymbol{\mathcal{A}} $$$ to be low-rank, which can be factorized as $$$ \boldsymbol{\mathcal{A}}_{(1)}=\mathbf{U} \mathbf{\Phi} $$$, where $$$ \boldsymbol{\mathcal{A}}_{(1)} $$$ is the mode-1 matricization of $$$ \boldsymbol{\mathcal{A}} $$$, $$$ \mathbf{U} $$$ contains spatial coefficient maps, and $$$ \mathbf{\Phi} $$$ contains matricized, separable multi-dimensional temporal basis functions describing T1/T2 relaxation, cardiac motion, and respiratory motion1. In reconstruction, $$$ \mathbf{\Phi} $$$ was first determined by the HOSVD of low-rank tensor–completed training data. $$$ \mathbf{U} $$$ was then recovered by fitting $$$ \mathbf{\Phi} $$$ to the acquired imaging data $$$ \mathbf{d} $$$, using the following optimization problem:$$ \mathbf{\hat{U}}=\text{arg}\underset{\mathbf{U}}{\operatorname{min}} ||\mathbf{d}-\Omega(\mathbf{FSU\Phi})||_2^2 + R(\mathbf{U}), $$ where $$$ \Omega $$$ is the undersampling operator, $$$ \mathbf{F} $$$ is the nonuniform Fourier transform operator, $$$ \mathbf{S} $$$ is the coil sensitivity operator, and $$$ R(\cdot) $$$ is an optional regularization functional to promote transform sparsity (chosen as a wavelet transform in this project).
T1,T2 Quantification: The native T1, T2 measurements are estimated from the signal model $$ s(A,B,T_1,T_2)=A \frac{1-e^{-TR/T_1}}{1-e^{-TR/T_1}cos(\alpha)} [1+(Be^{-\tau/T_2}-1)(e^{-TR/T_1}cos(\alpha))^n]\cdot sin(\alpha), $$ with amplitude factor $$$ A $$$, IR/T2-IR pulse efficiency $$$ B $$$, FLASH readout interval $$$ TR $$$, prescribed flip angle $$$\alpha$$$, and recovery time point $$$ n=1,2,..., N$$$, ( $$$ N $$$ is the number of FLASH pulses in a recovery period).
Results
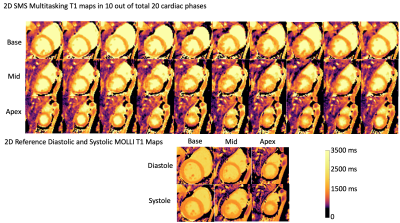
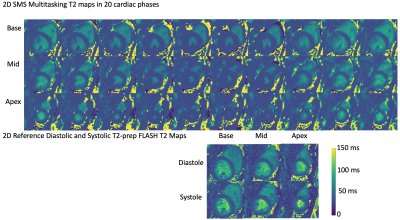
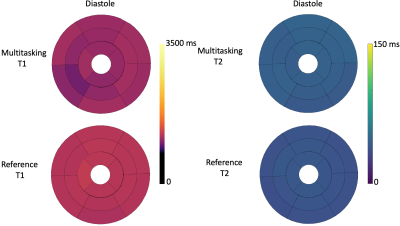
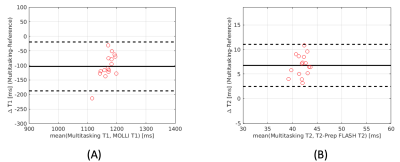
T1 and T2 cine mapping images acquired from the 2D SMS Multitasking sequence are shown in Fig.2 and Fig. 3, along with the reference 2D diastolic and systolic T1, T2 images acquired from MOLLI and T2-prep FLASH sequences. Fig. 4 depicts the summary statistics for segment-wise T1/T2 values in 7 human volunteers. Multitasking T1 maps reported slightly lower myocardial T1 values (1117±39.7ms) than the MOLLI T1 measurements (1221±16.8ms). Multitasking T2 maps produced slightly higher myocardial T2 values (45.3±1.8 ms) than the T2-prep FLASH T2 values (38.6±1.4 ms). Fig. 5 shows the Bland-Altman plot of the multitasking segment-wise T1/T2 values and reference myocardial T1/T2 values.Discussion and Conclusion
Co-registered T1 and T2 maps were simultaneously acquired at basal, mid, and apical slices using an SMS accelerated Multitasking technique in a 3-min scan. The myocardial T1, T2 quantification showed slight bias compared to reference MOLLI and T2-prep FLASH measurements, but were still in the reported normal range8. This method shows potential for reducing exam time for quantitative CMR without ECG or breath-holds. Future studies will focus on further reduction in scan time, validation in a larger cohort, repeatability assessments, and patient studies.Acknowledgements
This work was supported by NIH 1R01EB028146.References
[1] Christodoulou, A. G., Shaw, J. L., Nguyen, C., Yang, Q., Xie, Y., Wang, N., & Li, D. (2018). Magnetic resonance multitasking for motion-resolved quantitative cardiovascular imaging. Nature biomedical engineering, 2(4), 215-226.
[2] Mao, X., Serry, F. M., Ma, S., Hu, Z., Han, F., Xie, Y., L, D., & Christodoulou, A. G., “3D Free-Breathing, Non-ECG, T1-T2-B1+ Cine Mapping with Cardiac MR Multitasking,” in Proceedings of Society of Magnetic Resonance Angiography (SMRA), 32nd Annual International Conference, 2020.
[3] Mao, X., Serry, F. M., Cokic, I., Ma, S., Hu, Z., Han, F., Lee., H., Xie, Y., L, D., & Christodoulou, A. G., “3D Free-Breathing, Non-ECG, T1-T2-B1+ Cine Mapping with Cardiac MR Multitasking,” submitted to Society for Cardiovascular Magnetic Resonance (SCMR), 24th Annual International Conference, 2021.
[4] Barth, M., Breuer, F., Koopmans, P. J., Norris, D. G., & Poser, B. A. (2016). Simultaneous multislice (SMS) imaging techniques. Magnetic resonance in medicine, 75(1), 63-81.
[5] Weingärtner, S., Moeller, S., Schmitter, S., Auerbach, E., Kellman, P., Shenoy, C., & Akçakaya, M. (2017). Simultaneous multislice imaging for native myocardial T1 mapping: improved spatial coverage in a single breath‐hold. Magnetic resonance in medicine, 78(2), 462-471.
[6] Ye, H., Cauley, S. F., Gagoski, B., Bilgic, B., Ma, D., Jiang, Y., ... & Setsompop, K. (2017). Simultaneous multislice magnetic resonance fingerprinting (SMS‐MRF) with direct‐spiral slice‐GRAPPA (ds‐SG) reconstruction. Magnetic resonance in medicine, 77(5), 1966-1974.
[7] Zhao, B., Bilgic, B., Adalsteinsson, E., Griswold, M. A., Wald, L. L., & Setsompop, K. (2017, July). Simultaneous multislice magnetic resonance fingerprinting with low-rank and subspace modeling. In 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC) (pp. 3264-3268). IEEE.
[8] Von Knobelsdorff-Brenkenhoff, F., Prothmann, M., Dieringer, M. A., Wassmuth, R., Greiser, A., Schwenke, C., ... & Schulz-Menger, J. (2013). Myocardial T1 and T2 mapping at 3T: reference values, influencing factors and implications. Journal of Cardiovascular Magnetic Resonance, 15(1), 53.
Figures