3609
Carotid T1 mapping with Muscle Referenced B1 Mapping Correction Using Variable Flip Angle Imaging without Extra Scan
Huiyu Qiao1, Shuo Chen1, Zihan Ning1, Hualu Han1, Rui Shen1, and Xihai Zhao1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine Tsinghua University, Beijing, China
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, School of Medicine Tsinghua University, Beijing, China
Synopsis
Carotid T1 mapping calculated by variable flip angle imaging usually needs an additional B1 mapping to correct nominal flip angle. However, B1 mapping techniques are difficult to implement in clinical settings. This study proposed a muscle referenced B1 mapping for a more accurate carotid T1 mapping using variable flip angle imaging but without an additional B1 mapping scan. The proposed muscle referenced B1 mapping could effectively adjust the B1 inhomogeneity, particularly for the B1 inhomogeneity between left and right carotid vessel walls. Furthermore, the T1 mapping with muscle referenced B1 mapping correction showed the capability of identifying the intraplaque hemorrhage.
Introduction
Carotid T1 mapping has the potential capability of assessing vulnerable plaque components, particularly for intraplaque hemorrhage1. Coolen et al proposed a 3D carotid T1 mapping by combining improved motion-sensitized driven equilibrium (iMSDE) prepulse with variable flip angle imaging2. However, an additional B1 mapping scan needs to be performed for acquiring the actual flip angle in T1 mapping estimation using variable flip angle imaging. Clinically, B1 mapping techniques are difficult to implement. Hence, some studies proposed the method using a single tissue such as fat to determine the B1 mapping3. Anatomically, carotid arteries are surrounded by muscles. We hypothesized that carotid B1 mapping can be estimated using surrounding muscles as reference. The objective of this study was to propose carotid T1 mapping with muscle referenced B1 mapping correction based on the variable flip angle imaging but without the need of additional B1 mapping scan.Methods
Theory: The equation of calculating signal intensity using RF spoiled gradient echo (SPGR) with a nominal flip angle is as following: $$Signal=M_0\frac{(1-E1)sin\alpha}{(1-E1cos\alpha)}e^{-iMSDE_{dur}/T2},E1=e^{-TR/T1}$$Apparent T1 mapping (T1app) based on the nominal flip angle is evaluated using linear least square method. Actual T1 mapping (T1act) which depends on the B1 field can be simply expressed as T1app/B12. According the previous reports on the measurements of T1act of neck muscles, the B1 mapping can be calculated using the square root of T1app/T1act in the region of neck muscles. Since B1 mapping varies smoothly and carotid arteries are surrounded by muscles, it is possible to estimate carotid B1 mapping by interpolating the muscular B1 mapping.Volunteer experiments: Five healthy subjects (46.6±9.6 yrs, 3 males) and one atherosclerotic patient (74 yrs, male) were recruited in this study. Variable flip angle imaging was performed for all subjects within 4’22’’ on a 3.0 T MR scanner (Ingenia, Philips Healthcare, Best, the Netherlands) with a dedicated 8-channel carotid coil. The imaging parameters of variable flip angle imaging were as follows: iMSDE prepared SPGR, TFE factor 60/200; flip angle 18°/3°, TR/TE 8.0/3.2 ms, FOV 160×160×40 mm3, resolution 0.8×0.8×2 mm3.
In vivo data analysis: The flowchart of in-vivo data analysis for the T1 and B1 mapping calculation is shown in Figure 1. First, the images from two sequences were registered to minimize the effect of vascular pulsation using the open source Elastix image registration software4. The registration was performed in 3D using a nonrigid B-spline transformation model (a control point spacing of 15 mm) and mutual information as a similarity measure in a carotid region which is 15 mm around the center line of carotid artery5. The carotid local T1 mapping without B1 correction (T1app mapping) was calculated using local registered images. Second, muscular masks were manually drawn on 3° magnitude images using ImageJ software6. The B1 mapping on muscles was generated with the square root of muscular T1app mapping divided by T1act value (1000 ms)7. B1 mapping of each pixel in the carotid region was represented using the mean B1 value of the region which is 30 mm around the pixel. Third, carotid local T1 mapping with B1 correction (T1act mapping) was estimated using carotid local B1 mapping and local registered images. Finally, the carotid lumen and wall boundaries were manually outlined on the 3° magnitude images using the CASCADE software (UW, Seattle, Washington, USA). The masks of lumen and wall were exported and overlaid on T1app or T1act mapping. The T1 values on the carotid vessel wall were measured and recorded.
Statistical analysis: The continuous variables were described as mean ± standard deviation. T1app and T1act values were compared between left and right carotid vessel wall using Mann-Whitney U test.
Results
The T1app value and T1act value of carotid vessel walls were 1095.8±203.5 ms and 1197.9±99.5 ms, respectively (Figure 2). Significant difference in T1app values was found between left and right carotid vessel wall (1139.1±241.8 ms vs. 1053.3±207.2 ms, P<0.0001). There was no significant difference in T1act values between left and right carotid vessel wall (1205.7±143.8 ms vs. 1191.5±132.5 ms, P=0.139). Figure 3 represented the 3° and 18° magnitude images, T1 mapping with B1 correction and the multi-contrast vessel wall images of the same slice from the patient, in which intraplaque hemorrhage detected by multi-contrast vessel wall images showed shorter T1act values on T1 mapping images.Discussion and Conclusion
This study calculated the carotid T1 mapping with muscle referenced B1 mapping correction only based on the variable flip angle imaging but without the need of additional B1 mapping scan. Our work improves the clinical applicability of carotid T1 mapping using variable flip angle imaging by eliminating additional B1 mapping scan. It has been proved that muscle referenced B1 mapping could effectively adjust the B1 inhomogeneity on carotid vessel walls, particularly for the B1 inhomogeneity between left and right carotid vessel walls. In addition, the T1 mapping with muscle referenced B1 mapping correction showed the capability of identifying intraplaque hemorrhage. However, there is too much manual operation in the data analysis. A more automated process is warranted in the future.Acknowledgements
None.References
- Qiao H, Li D, Cao J, et al. Quantitative evaluation of carotid atherosclerotic vulnerable plaques using in vivo T1 mapping cardiovascular magnetic resonance: validation by histology. J Cardiovasc Magn Reson. 2020;22:38.
- Coolen BF, Poot DH, Liem MI, et al. Three-dimensional quantitative T1 and T2 mapping of the carotid artery: Sequence design and in vivo feasibility. Magn Reson Med. 2016;75:1008-1017.
- Pineda FD, Medved M, Fan X, Karczmar GS. B1 and T1 mapping of the breast with a reference tissue method. Magn Reson Med. 2016;75:1565-1573.
- Klein S, Staring M, Murphy K, Viergever MA, Pluim JPW. Elastix: a toolbox for intensity‐based medical image registration. IEEE Trans Med Imaging. 2010;29:196-205.
- Van'T Klooster R, Staring M, Klein S, et al. Automated registration of multispectral MR vessel wall images of the carotid artery. Med Phys. 2013;40:121904.
- Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671-675.
- Qi H, Sun J, Qiao H, et al. Simultaneous T 1 and T 2 mapping of the carotid plaque (SIMPLE) with T 2 and inversion recovery prepared 3D radial imaging. Magn Reson Med. 2018;80:2598-2608.
Figures
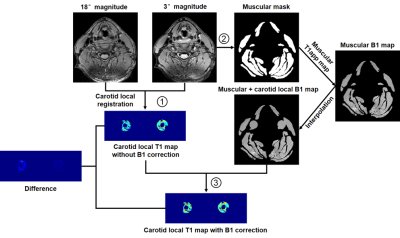
Figure
1. The
flowchart of in-vivo data analysis for the T1 and B1 mapping calculation.
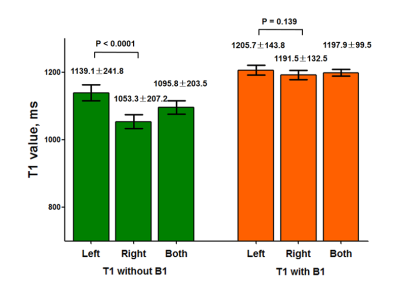
Figure 2. The
T1 values of left, right and both carotid vessel walls on the T1 mapping
without or with B1 correction.
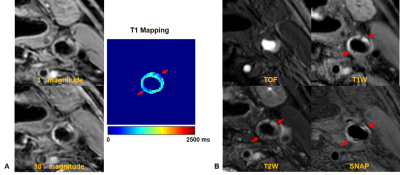
Figure 3. The
3° and 18° magnitude images, T1 mapping with B1 correction (A) and the
multi-contrast vessel wall images (B) of the same slice from a 74 years old
atherosclerotic patient.