3602
Multi-Echo GRASP for Cardiac T2*-Relaxometry1Department of Radiology, Medical Physics, Faculty of Medicine, University Medical Center Freiburg, University of Freiburg, Freiburg, Germany, 2University Heart Center Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 3German Consortium for Translational Cancer Research Partner Site Freiburg, German Cancer Research Center (DKFZ), Heidelberg, Germany
Synopsis
Iron accumulation is a side effect from regular blood transfusions, which are a common treatment for diseases like hemochromatosis and sickle cell disease. T2* mapping can be a useful tool in therapy planning as it allows for monitoring of the iron accumulation in the heart. Multi-echo-GRASP offers a method of T2* mapping for all phases of the cardiac cycle. The acquisition can be performed in free breathing which is beneficial for patients with an impaired cardiac and/or respiratory capacity.
Introduction
Iron accumulation is a side effect from regular blood transfusions, which are a common treatment for diseases like hemochromatosis and sickle cell disease. T2* mapping can be a useful tool in therapy planning as it allows for monitoring of the iron accumulation in the heart1. Additionally, new contrast agents are under development that bind to inflamed endothelial cells – these agents use iron oxide microparticles (MPIO) to create a negative MRI contrast due to a decrease in T2*2. However, reliable T2* relaxometry suffers from cardiac and respiratory motion leading to decreased sensitivity for small T2* changes. In this work, we propose a multi-echo GRASP3 technique to reduce the influence of cardiac and respiratory motion in cardiac T2* mapping.Methods
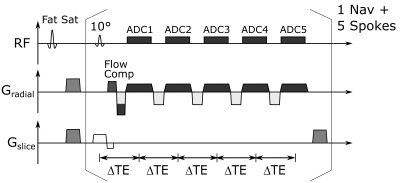
For T2* mapping a radial multi-echo sequence was implemented at a clinical 3T MRI system (Magnetom PRISMA, Siemens). The sequence uses flow compensation in readout direction and acquires 5 multi-echo trains with 5 echoes each (Fig. 1). Data acquisition is preceded by fat saturation and a respiratory navigator (non-selective excitation and readout in cranio-caudal direction). The following imaging parameters were used: TE = 3, 6, 9, 12, 15 ms, FA = 10 °, TR = 18.3 ms, FOV = 192x192 mm2, 1x1x5 mm3 resolution, TA = 2 min 27 s. Both flip angle and TR of the navigator were the same as in the subsequent imaging acquisitions to maintain the steady state. Radial trajectories were rotated by 111.25° (golden angle) after each echo train to cover k-space. In total, 5000 echo trains were acquired. For binning of the data along the cardiac cycle the time to the R-wave was recorded for each readout. Cardiac cycles which deviated by more than 20% from the mean R-R interval were excluded to prevent binning errors from incorrect cardiac triggering.Reconstruction was performed using a modified version of the reconstruction shown in Ref. 3. The acquired data was binned into 16 cardiac and four respiratory phases. An iterative reconstruction using a total variation regularization along both cardiac and respiratory dimension was used to reduce radial undersampling artifacts. Coil sensitivity maps were calculated from regridded k-space data using the BART toolbox4. The results were compared to a conventional black blood multi-echo GRE sequence acquired during one breath-hold (TE=3.5, 7.6, 12.75, 17.8 ms, TR = 350 ms, FOV = 192x192 mm2, 1x1x5 mm3 resolution, TA = 16-19 s). T2* values were calculated from a mono-exponential fit of the signal magnitude vs. the echo time. Initial in vivo experiments were performed in two healthy volunteers (both male, 30y) and in a pig model under general anesthesia and external ventilation. With an RR interval of 1000ms and and 12 segments per heartbeat, the reference measurement was done in 16 cycles, while the GRASP sequence was running continuously. The imaging slice was positioned in a short axis view at the mid-ventricular level.
Results
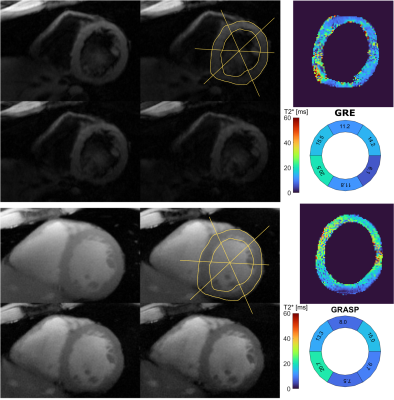
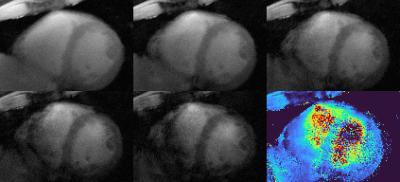
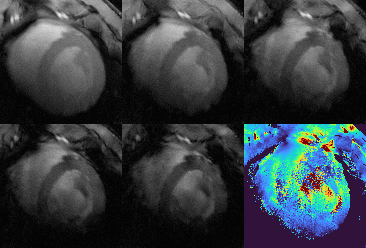
The mean myocardial T2* was T2*GRE=13.2 ms and T2*GRASP=12.3 ms for GRE reference and GRASP respectively. Figure 2 shows the results of the 2D GRASP technique in comparison to the reference measurement in one volunteer. Four anatomical images and the T2* map of the segmented myocardium are shown: the T2* values exhibit a mean deviation of 24% over all six sextants of the bullseye plots when compared to the reference values in an exemplary cardiac cycle during diastole. In Figures 3 and 4 the T2* map as well as the anatomical image are animated along the cardiac cycle for the human (Fig. 3) and porcine measurement (Fig.4). Reconstruction time was 3 h 30 min for one complete multi-echo GRASP acquisition on a standard workstation.Discussion
Results show that T2* maps can be reliably measured with the proposed technique. Even though the acquisition duration is 8 times as long than a conventional sequence acquired in a breath hold, in clinical settings the GRASP sequence is preferable as a 19-second long breath hold might not be feasible for patients with chronic cardiac diseases. Furthermore, the GRASP technique yields both anatomical cine images as well as the T2* maps within one acquisition. The long reconstruction times are currently impeding a clinical routine application, but they can be reduced by a GPU acceleration of the reconstruction code. The acquisition efficiency could be increased by extracting the cardiac phase from the navigators using additional components of the PCA as the ECG-signal may be impaired by noise induced by gradient switching.Conclusion
Multi-echo-GRASP offers a method of T2* mapping for all phases of the cardiac cycle. The acquisition can be performed in free breathing which is beneficial for patients with an impaired cardiac and/or respiratory capacity.Acknowledgements
Grant support by the German Science Foundation (DFG) under grant number BO 3025/11-1 and CRC 1425 (Project P15) is gratefully acknowledged.References
1Triadyaksa P, Oudkerknand M, Sijens P, “Cardiac T2* Mapping: Techniques and Clinical Applications". J Magn Reson Imaging (2019) doi:10.1002/jmri.27023
2Heidt T, Reiss S, Lottner T, Özen A, Bode C, Bock M, von Zur Mühlen C, "Magnetic resonance imaging for pathobiological assessment and interventional treatment of the coronary arteries", Eur Heart J Suppl. (2020) 22(Suppl C):C46-C56 doi:10.1093/eurheartj/suaa009
3Feng L, Grimm R, Block KT, Chandarana H, Kim S, Xu J, Axel L, Sodickson DK, Otazo R. "Golden-angle radial sparse parallel MRI: combination of compressed sensing, parallel imaging, and golden-angle radial sampling for fast and flexible dynamic volumetric MRI". Magn Reson Med. 72(3):707-17 (2014) doi:10.1002/mrm.24980
4BART Toolbox for Computational Magnetic Resonance Imaging, DOI: 10.5281/zenodo.592960Figures