3601
Evaluation of various image descriptor for motion correction between pre- and post-injected cardiac T1 maps, based on a demon algorithm1Research & Innovation, Olea Medical, La Ciotat, France
Synopsis
The registration of pre- and post-injected T1 maps is a multimodal image registration problem. To employ algorithms based on intensity preservation assumption, one need to use image descriptor. By comparing six of them, we aim to choose the most adequate one to our issue. We conducted our analyzes using a STEMI-patients database. The Sobel filter emerge as the most accurate and precise descriptor. The study of the results shows that the position of the acquired slice has no effect on the performances of the algorithm, while the size of the lesion and the cross-slice motion are inversely correlated to them
Introduction
The motion correction between pre- and post-injection cardiac T1 maps is implemented to estimate the extracellular volume (ECV)1. The use of algorithms based on intensity preservation assumption requires the employment of image descriptors. In this paper, we propose comparing and analyzing six of them.Methods
The original images used for the registration are the images with the higher inversion time (TI) from the modified Look-Locker inversion recovery (MOLLI) sequence, and this to reduce the effect of noise observed in the T1 maps and to use the closest images in term of variation of intensities between structures (closest to the steady-state and then less sensitive to T1*).We used the data of a 73 STEMI-patients database. Five slices were scanned for each patient, and an expert manually delineates the myocardium for 1 to 3 slices each time, for a total of 148 labeled slices.
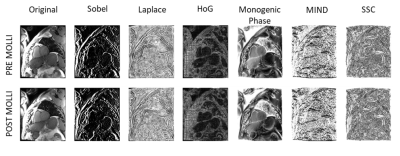
The descriptors used (figure 1) are the original images, the gradient magnitude computed using a Sobel-based filter, a Laplacian filter, the gradient in its median direction using histogram of oriented gradient (HOG), the phase of the monogenic signal (monogenic phase), the modality independent neighborhood descriptor in the 4-chamber direction plane (MIND), and the magnitude of the self-similarity context (SSC)2–5.
The registration step is driven using the fast symmetric and diffeomorphic demon, with a coarse-to-fine strategy with three Gaussian pyramid levels: shrink factor (4, 2, 1); SD (6, 4, 2)6. The SD of the Gaussian smoothing of the displacement field was fixed at 4.3 mm and the maximum iteration to 50.
To evaluate the registration results, we used the Dice metric and the Hausdorff distance (HD) between the myocardium masks and the normalized mutual information (NMI) between images. We also analyzed the results in terms of slice position, cross-slice-motion identified using the relative volume difference (RVD) between the myocardium masks, and size of infarct lesion in percentage of the myocardium7.
Results
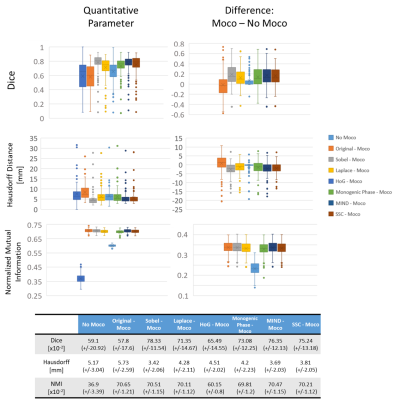
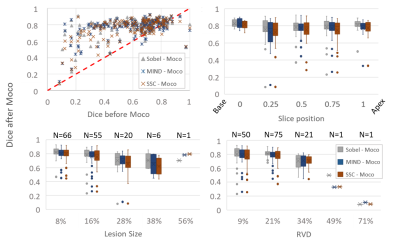
The global results are shown in figure 2. The more accurate matching (average: Dice>0.75, HD<4mm) was achieved using the Sobel, MIND and SSC approaches, with an advantage for the Sobel filter in terms of matching and precision (SD: Dice +/-0.12, HD +/-2.06mm). We also observed a reduction of the myocardium matching after registration in some cases. However, the NMI was increased for all the slices.Figure 3 details the Dice results for the Sobel, MIND and SSC approaches. The comparison of Dice before and after motion correction revealed that all the decreases in matching occurred for initially registered myocardium. Indeed, the lower Dice before motion correction for this subgroup is 0.66, 0.68, and 0.69 respectively for MIND, SSC, and Sobel. No relationship between the median Dice and the slice positions can be reported here (R2<0.15). But a coefficient of determination of at least 0.90 between Dice and RVD was obtained for all the approaches. The lesion size case also revealed a good correlation (R2=0.72) for the Sobel approach, but only if the 56% value is excluded for MIND and SSC (respectively R2=0.07/0.03 to 0.99/0.97). The matching obtained after motion correction between myocardium decreases with the increase of RVD or lesion size.
Considering a Dice < 0.6 after motion correction as a failure case, the results revealed that this happened 10, 13, and 13 times respectively for Sobel, MIND, and SSC, for 148 processed cases. The worst case was obtained for a RVD of 71%. Other cases that cannot be explained by high RVD were explored visually and the authors conclude to a cardiac phase mismatch.
Discussion & Conclusion
The systematic increase of the NMI suggests an improvement of the matching on the whole domain of the image. However, the heart occupies a small part of it, and this improvement does not always occur for the myocardium. The fact that a decrease of the pre- and post-myocardium match occurred only for well enough registered images highlights the need of a registration assessor technique.The pre- and post-injected MOLLI acquisitions are spaced more than 10 min apart. The period during which the patient can breathe freely. The heart’s relative position between two different breath holds at a similar slice position could then be different. This should be detectable based on the RVD since the observed volume of the myocardium varies. We expect a reduction in the algorithm’s performance in such case. And, indeed, we observed such a behavior. This plaids for both 3D acquisition and registration of the heart.
All the descriptors used are based on a local similarity measure. The presence of a lesion in the post-MOLLI images can complicate the registration. And this is what we observe when we compare the algorithms’ performance to the lesion size. To reduce its effect, one strategy could be to homogenize the myocardium artificially, which require the procurement of the myocardial structure.
The cardiac phase mismatch leaded to a decrease in the performance of our algorithms. A potential strategy to solve the problem would be to apply the motion information for the strain computation to homogenize the cardiac phase between pre- and post-MOLLI images.
Finally, all the results plaids in favor of the Sobel descriptor for the motion correction between the pre- and post-injected T1 maps.
Acknowledgements
The authors thank Charles de Bourguignon for providing the epi and endocardial contours used for this study.References
1. Messroghli, D. R. et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 19, 75 (2017).
2. Dalal, N. & Triggs, B. Histograms of oriented gradients for human detection. in 2005 IEEE Computer Society Conference on Computer Vision and Pattern Recognition (CVPR’05) vol. 1 886–893 vol. 1 (2005).
3. Bridge, C. P. Introduction To The Monogenic Signal. ArXiv170309199 Cs (2017).
4. Heinrich, M. P. et al. MIND: Modality independent neighbourhood descriptor for multi-modal deformable registration. Med. Image Anal. 16, 1423–1435 (2012).
5. Heinrich, M. P., Jenkinson, M., Papież, B. W., Brady, S. M. & Schnabel, J. A. Towards Realtime Multimodal Fusion for Image-Guided Interventions Using Self-similarities. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2013 (eds. Mori, K., Sakuma, I., Sato, Y., Barillot, C. & Navab, N.) 187–194 (Springer, 2013). doi:10.1007/978-3-642-40811-3_24.
6. Vercauteren, T., Pennec, X., Perchant, A. & Ayache, N. Symmetric Log-Domain Diffeomorphic Registration: A Demons-Based Approach. in Medical Image Computing and Computer-Assisted Intervention – MICCAI 2008 (eds. Metaxas, D., Axel, L., Fichtinger, G. & Székely, G.) 754–761 (Springer, 2008). doi:10.1007/978-3-540-85988-8_90.
7. Yeghiazaryan, V. & Voiculescu, I. Family of boundary overlap metrics for the evaluation of medical image segmentation. J. Med. Imaging 5, (2018).
Figures