3596
Imaging pH, metabolism and hypoxia using hyperpolarized 13C-MRI and [18F]FMISO-PET to predict NIS expression in MSC gene therapy in glioblastoma1Department of Nuclear Medicine, Klinikum rechts der Isar, Technical University of Munich, School of Medicine, Munich, Germany, 2Medizinische Klinik und Poliklinik IV-Campus Großhadern, University Hospital of Munich, Ludwig-Maximilians-University Munich, Munich, Germany, 3Department of Pathology, Klinikum rechts der Isar, Technical University of Munich, Munich, Germany, 4Medizinische Klinik und Poliklinik IV, University Hospital of Munich, Ludwig-Maximilians-University Munich, Munich, Germany, 5Neurosurgical Research University Clinics, Ludwig-Maximilians-University Munich and Walter-Brendel-Centre of Experimental Medicine, Munich, Germany
Synopsis
A novel treatment approach for glioblastoma is based on mesenchymal stem cell (MSC)-mediated gene therapies whereby cell accumulation can be influenced by the tumor microenvironment. Here, we demonstrate the ability to image pH, metabolic pyruvate-lactate conversion and hypoxia in glioblastoma using hyperpolarized [1,5-13C2,3,6,6,6-D4]zymonic acid-MRSI, [1-13C]pyruvate-MRI and [18F]FMISO-PET as predictors for hypoxia-targeted sodium-iodide-symporter (NIS)-expression of tumor-infiltrating MSCs assessed by 124I-PET. Observed hypoxia (SUVmean = 0.49±0.05) was confirmed by histology and occurred together with increased lactate-production (AUCmean = 1.14±0.17) and mild acidification (pHmean = 7.34±0.02). This shows to be a suitable environment for NIS-MSC-activity, thereby allowing efficient therapy.
Introduction
Glioblastoma multiforme is a highly malignant and heterogeneous tumor of the central nerve system. Despite the large variety of existing treatment approaches1, prognosis and therapy outcome are still devastating. A promising novel therapy approach uses hypoxia-induced activation of sodium-iodide-symporter (NIS), mediated by mesenchymal stem cells (MSC)2 which allows theranostics using radioactive iodine isotopes3,4. The efficacy of this gene therapy concept depends on accumulation of MSCs and expression of NIS within the tumor which might be driven by hypoxia and tumor metabolism. Here, we studied the ability for tumor characterization using multimodal imaging of pH5,6, metabolic pyruvate-lactate conversion and hypoxia using hyperpolarized 13C-MR(S)I and 18F-PET and investigated how hypoxia and increased lactate production influence NIS-expression in MSCs within the tumor.Methods
Study Size: 10 mice (CD-1 nu/nu) each were injected 1∙106 patient-derived glioblastoma cells (GBM2) into the right flank.Hyperpolarization: For pH-imaging 27mg [1,5‑13C2,3,6,6,6-D4]zymonic acid (ZAd) and 25mg 13C-urea were co-polarized at 1.2K using a Hypersense DNP Polarizer (Oxford Instruments). Dissolution was performed using TRIS-buffered D2O resulting in a final concentration of 50mM ZAd and 75mM 13C-urea. To image metabolism, 25mg [1‑13C]pyruvate was polarized and dissolved with TRIS-buffered H2O to 80mM final concentration.
Imaging Hardware: MR-experiments were performed in a small animal 7T magnet (Agilent/GE) MR901 with Bruker AVANCE III HD electronics and a 31mm inner diameter 13C/1H-volume resonator (RAPID Biomedical). PET-Acquisitions were performed on a preclinical PET/CT (Inveon, Siemens).
Protocol: Imaging modalities were used and injections were performed as shown in Fig. 1a.
MR-Imaging + Hyperpolarized MRSI/MRI: Tumors and a [1‑13C]lactate‑phantom for B1-calibration were covered with carbomer gel (Carbopol 980, Caelo) for shimming and located using T2-weighted RARE-imaging. Diffusion-weighted-imaging (DWI) was performed using a DWI-EPI-sequence with 16 b-values. Hyperpolarized MRSI for pH-imaging was performed using FIDCSI with FA 15°, matrix size 14x14, slice thickness 5mm, FOV 28x28mm2, spectral bandwidth 3201Hz, 256 points. Hyperpolarized MRI for metabolic conversion was performed using a 3D B1-alternating bSSFP-sequence7 with FAPyr = 4°, FALac = 90°, matrix size 32x16x14, FOV 56x28x24.5mm3, temporal resolution 1.05s, 180 repetitions.
MSC-application: 5∙105 human bone marrow-derived mesenchymal stem cells, stably transfected with a synthetic hypoxia-responsive promoter driving NIS-expression, were tail vein-injected.
PET-Imaging: Mice were injected 12.71±0.78 MBq [18F]Fluoromisondazole ([18F]FMISO) and 10.6±0.6 MBq [124I]NaI. Acquisitions of [18F]FMISO- and [124I]iodine-PET started three hours post injection lasting one hour or 20 minutes respectively. Image reconstructions with 3D-OSEM used matrix size 128x128x159, voxel size 0.8x0.8x0.8mm3.
Data Processing: MR‑imaging data was analyzed in MatLab (MathWorks), zero-filled by a factor of four and pH-maps were calculated as previously reported5. Pyruvate-to-lactate conversion was quantified using area-under-the-curve (AUC)-ratios8. Standard Uptake Values (SUV) were calculated from activity concentration, injected activity and body weight. Apparent diffusion coefficients (ADC) were fitted monoexponentially. The mean values of ADC, AUC, pH and SUV were calculated across slice-wise drawn region-of-interests (ROI) and composed to 3D-ROIs if applicable.
Pathology: Mice were administered 60mg/kg pimonidazole one hour before tumor removal. (FFPE) Tumor tissue was sliced according to MR-image-orientation and stained for pimonidazole and carbonic anhydrase 9 (CAIX).
Results
ADC-maps (Fig. 1c) of subcutaneous tumors (Fig. 1b) show homogeneously dense tissue with ADCmean = ((1.01±0.03) , n=10) while pH-maps (Fig. 1d) reveal heterogeneity including acidified hotspots and light overall tumor acidification pHmean = (7.34±0.02, n=10). Imaging of lactate (Fig. 2a) and pyruvate (Fig. 2b) shows strong, heterogeneous lactate production (Fig. 2c) quantified by AUCmean = (1.14±0.17, n=8). No general correlation between acidification and pyruvate-lactate metabolism can be observed (Fig. 5a). [18F]FMISO-PET indicates increased tumor uptake with SUVmean = (0.49±0.05, n=7) compared to muscle tissue (SUVmuscle = 0.11±0.01, n=7) (Fig. 3a), correlating strongly spatially (comp. Fig. 2a) and intertumoral (Fig. 5b) with increased lactate production while not correlating with tumor acidification (Fig. 5c).Two days after MSC-injection, the hypoxia-driven NIS-mediated tumoral radioiodine accumulation was imaged by 124I-PET. PET-images reveal heterogeneous uptake (Fig. 3b) within the tumor, correlating strongly with [18F]FMISO-Uptake (Fig. 3c). Immunohistochemistry staining coregistered with MRI (Fig. 4a) for pimonidazole (Fig. 4b) confirms the presence of hypoxia and strong expression of CAIX (Fig. 4c).
Discussion
The observations suggest that in the glioblastoma model under investigation, anaerobic glycolysis is used to overcome hypoxia even though the Warburg effect9, favoring this metabolic pathway even under normoxic conditions, cannot be fully excluded. Extracellular buffer capacity appears high enough such that produced lactate does not or only mildly acidify the extracellular tumor microenvironment, whereas under normoxic conditions, overexpression of CAIX might lead to stronger extracellular acidification10. Despite imaging-based hypoxia and metabolic conversion assessment appears to be predictive for MSC-mediated hypoxia-induced NIS gene therapy, other influencing factors such as cytokines, lacking imaging modalities, must be considered. The observations should also be validated in orthotopic models to verify clinical transferability. Nevertheless, this study provides additional insight, since the blood-brain barrier did not limit the delivery of multimodal agents in the subcutaneous model.Conclusion
We demonstrated a multimodal imaging characterization of a patient-derived glioblastoma model in mice regarding pH, metabolic pyruvate-to-lactate conversion and hypoxia. Low acidification and strong hypoxia appear to be a favorable environment for induction of hypoxia-responsive NIS-activity in MSCs. Both parameters can be assessed with the described imaging modalities, therefore suggesting their use as predictive imaging biomarkers in the context of NIS-based gene therapy concepts.Acknowledgements
We acknowledge help from Sibylle Reder, Markus Mittelhäuser and Hannes Rolbieski for help with PET-Acquisitions, Michael Herz for PET-Tracer Synthesis and Marion Mielke, Olga Seelbach und Tanja Groll from pathology department (CeP Steiger) for help with histology. Further, we acknowledge support from the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation – 391523415, SFB 824).References
1. Anjum K, Shagufta B I, Abbas S Q, et al. Current status and future therapeutic perspectives of glioblastoma multiforme (GBM) therapy: A review. Biomed Pharmacother 2017; 92:681-689
2. Al-kharboosh R, ReFaey K, Montserrat L-V, et al. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin Proc Inn Qual Out 2020; 4(4):443-459.
3. Mueller A M, Schmohl K A, Knoop K, et al. Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery. Oncotarget 2016; 7(34):54795-54810.
4. Schug C, Gupta A, Urnauer S, et al. A Novel Approach for Image-Guided 131I Therapy of Pancreatic Ductal Adenocarcinoma Using Mesenchymal Stem Cell-Mediated NIS Gene Delivery. Mol Cancer Res 2018; 17(1): molcanres.0185.2018
5. Duewel S, Hundshammer C, Gersch M, et al. Imaging of pH in vivo using hyperpolarized 13C‑labelled zymonic acid. Nature Commun 8, 15126 (2017).
6. Hundshammer C, Duewel S, Koecher S, et al. Deuteration of Hyperpolarized 13C‐Labeled Zymonic Acid Enables Sensitivity‐Enhanced Dynamic MRI of pH. Chemphyschem. 2017; 18(18): 2422-2425.
7. Skinner J G, Topping G J, Heid I, et al. Fast 3D hyperpolarized 13C metabolic MRI at 7T using spectrally selective bSSFP. Digital Poster at ISMRM2020 International Conference 2020.
8. Hill D K, Orton M R, Mariotti E, et al. Model Free Approach to Kinetic Analysis of Real-Time Hyperpolarized 13C Magnetic Resonance Spectroscopy Data. PloS ONE 2013; 8(9): e71996
9. Warburg O, Posener K, Negelein E. Über den Stoffwechsel der Carcinomzelle. Biochemische Zeitschrift 1924; Band 152:309–344.
10. Lee S-H, Griffiths J R, How and Why are Cancers Acidic? Carbonic Anhydrase IX and the Homeostatic Control of Tumour Extracellular pH. Cancers 2020, 12, 1616
Figures
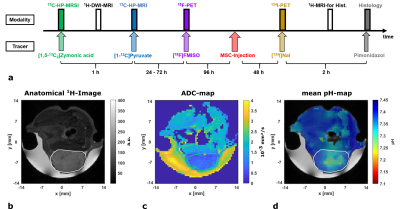
a: Imaging study protocol showing the temporal sequence of the applied modalities and injected tracers.
b: Axial anatomical T2-weighted 1H-RARE-image of a mouse bearing a subcutaneous GBM2-tumor (ROI encircled by white line) and Gd‑doped [1-13C]lactate-phantom (white arrow) covered by gel.
c: ADC-map showing the fitted diffusion coefficients based on 16 b-value images.
d: Mean pH-map weighted by compartment-intensities in the corresponding voxel overlaid with anatomical image.
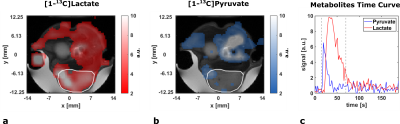
a: Axial [1-13C]lactate intensity image integrated over the range displayed in c overlaid with an anatomical image.
b: Axial [1-13C]pyruvate intensity image integrated over the range displayed in c overlaid with an anatomical image.
c: Signal time curves for [1-13C]pyruvate (blue) and [1-13C]lactate (red) with a 3D tumor ROI. Integration ranges for intensity images in a and b are displayed with dashed lines.
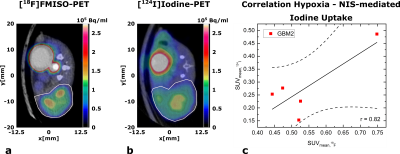
a: Axial image of [18F]FMISO-uptake overlaid with CT image. GBM2 tumors (ROI encircled by white line) show increased and heterogeneous uptake compared to healthy tissue (muscle around spine)
b: Axial image of [124I]iodine-uptake overlaid with CT image. GBM2 tumors (ROI encircled by white line) show heterogeneous uptake, partially correlating spatially with [18F]FMISO-Uptake in a.
c: Correlation plot of tumor SUVmean for [18F]FMISO and tumor SUVmean for [124I]iodine. Linear regression shows strong correlation between both parameters (r = 0.82).
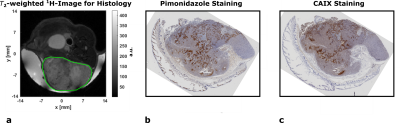
a: Axial anatomical T2-weighted 1H-RARE-image a subcutaneous GBM2-tumor (ROI encircled by green line) covered with gel. Image was acquired prior to tumor resection for coregistration with pathology.
b: Immunohistochemical staining for pimonidazole aggregation in the tumor. Tissue was sliced according to axial MR-image in a. Brown areas indicate positive staining.
c: Immunohistochemical staining for carbonic anhydrase 9 (CAIX) expression in the tumor. Tissue was sliced according to axial MR-image in a. Brown areas indicate positive staining.
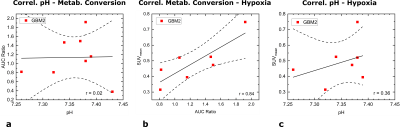
a: Correlation plot of tumor mean pH with tumor mean AUC ratio. Linear regression shows no correlation between both parameters (r = 0.02).
b: Correlation plot of tumor metabolic conversion rate AUC ratio and SUVmean for [18F]FMISO. Linear regression shows strong correlation between both parameters (r = 0.84).
c: Correlation plot of tumor mean pH and SUVmean for [18F]FMISO. Linear regression shows weak correlation between both parameters (r = 0.36), indicating lower pH values at lower [18F]FMISO-uptake.