3595
Metabolic Role of ATM in Development of Diffuse Large B-cell Lymphoma1Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 2Department of Anesthesiology, University of Maryland, Baltimore, MD, United States, 3Department of Medicine, University of Maryland, Baltimore, MD, United States, 4Veterans Administration Medical Center, Baltimore, MD, United States
Synopsis
Ataxia-telangiectasia mutated (ATM) kinase regulates critical metabolic processes of B-cell lymphoid malignancies. Diffuse large B-cell lymphoma (DLBCL) is clinically a very aggressive tumor subtype. This study investigates the role of ATM in metabolism. Oxygen consumption rate measurements showed decreased respiration in DLBCL cells lacking ATM. MRS of hyperpolarized [1-13C]pyruvate in DLBCL line HLY that were either wild-type or had ATM knocked down showed increased conversion of hyperpolarized pyruvate to lactate in HLY cells lacking ATM. These results indicate that deregulation of mitochondrial structure/function due to ATM deficiency results in metabolic changes and may contribute to the development of cancer.
Introduction
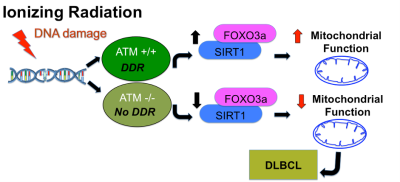
A mutation in Ataxia-telangiectasia mutated (ATM) gene contributes to the development of B-cell lymphoid malignancies. Diffuse large B-cell lymphoma (DLBCL) is clinically a very aggressive tumor subtype and is genetically heterogeneous. Previous reports and published data indicate that Forkhead Box O3 (FOXO3a) and NAD-dependent deacetylase sirtuin-1 (SIRT1) are regulated in an ATM-dependent manner in B-cells and HeLa cells.1 These observations suggest that ATM regulates metabolic pathways that are critical for the development of DLBCL. However, no metabolic role for ATM in regulating lymphoma growth has yet been established. This study investigates the role of ATM in metabolism by looking at mitochondrial homeostasis as well as measuring metabolism using hyperpolarized [1-13C]pyruvate.Materials and Methods
GM lymphocytes were used as a model for normal B cells. GM+/+ cells were acquired from a patient with the ATM gene while GM-/- lymphocytes were from a patient with an ATM deletion. HLY was used as a model for DLBCL. Lentiviruses were used to deliver short hairpin RNA against ATM in HLY cells (HLY sh- ATM(-/-)) or a non-targeted short hairpin RNA (HLY NT (+/+)). Mitochondrial morphology was measured using electron microscopy (EM) and oxygen consumption rate (OCR) was measured using SeaHorse (Agilent, MA).In vitro magnetic resonance spectroscopy (MRS) studies (n = 3 for each HLY cell line) were conducted utilizing a clinical 3 T GE 750w MRI scanner (GE Healthcare, Waukesha, WI, USA). Before each dissolution, approximately 1 × 107 cells were resuspended in 2 mL culture media. This was followed by an injection of 2 mL of approximately 14 mM hyperpolarized [1-13C] pyruvate solution. MR measurements were performed using a custom-built 13C surface coil (diameter = 28 mm) used for both radiofrequency (RF) excitation and signal reception. Spectra were acquired with non-selective RF pulse excitation with a small flip angle of 5.625° at every 3 s for 80 scans. Lactate-to-pyruvate ratios were calculated by summing the first 40 time-points (120 s) of each metabolite's time curve and taking ratios of the metabolite signal intensities.
Results
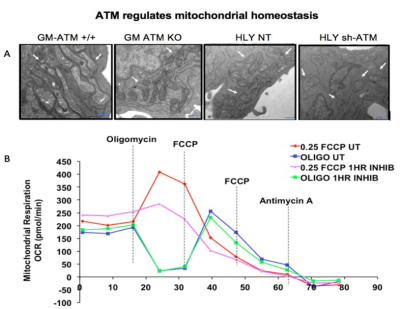
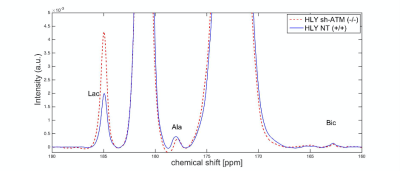
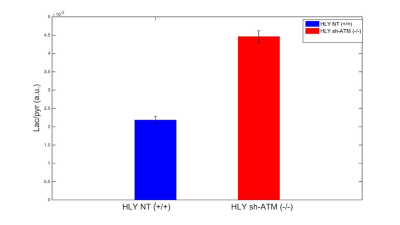
We performed EM of mitochondrial structure and OCR measurement of mitochondrial respiration. EM data shown in Fig. 2A demonstrated that ATM unmutated (wt) malignant B-cells consisted of mixed population of tubular and smaller mitochondria compared to typical bacillus-shaped mitochondria in normal B-cells. The most striking observation was that the outer membrane was fragmented and a significant lack of the cristae structure within the mitochondria of ATM-/- DLBCL cells. Mitochondria from ATM-inhibited DLBCL cells also displayed a much greater variation in width and were more swollen and rounded in shape. OCR was significantly reduced in HLY cells treated with the ATM inhibitor compared to untreated HLY cells (Fig. 2B). This led us to hypothesize that mitochondrial metabolism is compromised in presence of ATM deficiency.To confirm a change in metabolism, we utilized the MRS of hyperpolarized [1-13C]pyruvate. Figure 3 shows the comparison between lactate-to-pyruvate ratio level for HLY NT (+/+) and HLY sh-ATM (-/-) and indicates that HLY sh-ATM (-/-) produces more lactate compared to HLY NT (+/+). Figure 4 depicts the bar graph with standard error for the HLY NT (+/+) and HLY sh-ATM (-/-). The mean valve of lactate-to-pyruvate ratio was 2.18×10-3 ± 1.04×10-4 for HLY NT (+/+) and 4.46×10-3 ± 1.63 ×10-4 for HLY sh-ATM (-/-). These results from hyperpolarized 13C MRS confirm that ATM deficiency results in changes in cellular metabolism and produces more lactate from pyruvate.
Discussion and Conclusions
This study has shown a relationship between ATM, a protein involved in DNA damage, and mitochondrial metabolism. While previous studies have shown that ATM is involved in the regulation of several proteins important in metabolism, we demonstrated that ATM knockdown cells have impaired mitochondrial morphology and function. Furthermore, there is also a measurable difference in pyruvate flux when ATM is lost, as demonstrated by hyperpolarized [1- 13C]pyruvate MRS. The exact mechanisms controlling these changes in metabolism remain unknown, but deregulation of mitochondrial structure/function due to ATM deficiency may contribute to the development of cancer, particularly in the context of DLBCL. Delineating the metabolic crosstalk between ATM and DLBCL will help us to develop therapies specifically targeting metabolic dysregulation in lymphoma and other related disorders. In the future, we propose using hyperpolarized glutamine to directly probe metabolism in the tricarboxylic acid (TCA) cycle.Acknowledgements
This work was supported by NIH grants R01CA164311, R01 DK106395, R21 CA213020, R21 CA202694, R21 NS096575, a Merit Review Award from Department of Veterans Affairs, ACS grant award 10021596, UMMS Foundation Nathan Schnaper Fund.References
1. Mazan-Mamczarz, K. et al. ATM regulates a DNA damage response posttranscriptional RNA operon in lymphocytes. Blood 117, 2441–2450.
Figures