3594
Comparing aspartate and bicarbonate produced from hyperpolarized 1-13C pyruvate as markers of renal gluconeogenic flux
Hikari A. I. Yoshihara1, Arnaud Comment2,3, and Juerg Schwitter4,5
1Laboratory for Functional and Metabolic Imaging, Institute of Physics, Swiss Federal Institute of Technology, Lausanne (EPFL), Lausanne, Switzerland, 2Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 3General Electric Healthcare, Chalfont St Giles, United Kingdom, 4Division of Cardiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 5Cardiac MR Center, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
1Laboratory for Functional and Metabolic Imaging, Institute of Physics, Swiss Federal Institute of Technology, Lausanne (EPFL), Lausanne, Switzerland, 2Cancer Research UK Cambridge Institute, University of Cambridge, Cambridge, United Kingdom, 3General Electric Healthcare, Chalfont St Giles, United Kingdom, 4Division of Cardiology, Lausanne University Hospital (CHUV), Lausanne, Switzerland, 5Cardiac MR Center, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland
Synopsis
Labeled metabolites of hyperpolarized 1-13C pyruvate, including aspartate, malate and fumarate are detected in the rat kidney in vivo, indicating the possibility of detecting gluconeogenic flux. Pyruvate-to-bicarbonate conversion in the fasted rat liver is a marker of PEP-CK flux. Fasting resulted in lower bicarbonate and higher aspartate in the kidney. Conversely, treatment with the PEP-CK inhibitor 3-MPA did not affect bicarbonate production but did yield a lower normalized aspartate signal, with a 12-fold reduction in fasted rats. Renal pyruvate-to-bicarbonate conversion is therefore largely attributable to pyruvate hydrogenase flux, while pyruvate-to-aspartate conversion is a potential marker of renal gluconeogenesis.
Introduction
The kidney is both a consumer and producer of glucose, and these metabolic pathways are largely confined to different segments of the nephron. Renal gluconeogenic flux makes a significant contribution to total glucose production, and it is elevated in conditions such as diabetes.1 Hyperpolarized 1-13C pyruvate is incorporated into gluconeogenic pathways in the liver, detected by the metabolites downstream of pyruvate carboxylase (PC), most prominently aspartate. Additionally, bicarbonate produced from pyruvate in the livers of fasted rats and mice has been attributed to gluconeogenic flux via phosphoenolpyruvate carboxykinase (PEP-CK).2,3 Bicarbonate from pyruvate, however, generally results from pyruvate dehydrogenase (PDH) flux in most tissues. The same pyruvate metabolites seen in the liver can be detected in the kidney, and this study was performed to assess the contribution of gluconeogenic flux to the bicarbonate produced from hyperpolarized pyruvate in the kidney.Methods
Animal experimentsMale Wistar (n = 10, 255.5 ± 13.7 g) were anesthetized with isoflurane and fitted with catheters in the femoral arteries and vein. Blood was sampled from an arterial catheter and analyzed immediately. The fasted group was deprived of food overnight for ~12 hr. When used, the PEP-CK inhibitor 3-mercaptopicolinic acid (3-MPA) was administered intraperitoneally (100 mg/kg, neutralized in PBS) 1 hr prior to the hyperpolarized pyruvate infusion.
Pyruvate polarization
10 µl of 1-13C pyruvic acid formulated with OX63 trityl radical was frozen in a sample cup, along with 13 µl of 10 M NaOH for neutralization, and polarized in a custom-built instrument with microwave irradiation (196.59 GHz, 55 mW, 7 T and 1.05 K). The sample was dissolved with 5.5 mL of hot phosphate-buffered D2O, rapidly transferred to the scanner magnet, and 1.4 mL was infused intravenously.
MR acquisition
Data were acquired in a 9.4 T horizontal-bore magnet (Magnex) with a VNMRS console (Varian). A surface coil equipped with a single loop tuned to 1H and two 16 mm loops in quadrature tuned to 13C was placed over the left kidney (to avoid signals from the liver), its position verified by 1H GEMS MRI, and shimmed using FASTESTMAP. A series of 13C spectra were acquired starting with the pyruvate infusion (~2.9 s repetition time, with respiratory gating and cardiac triggering, 30º BIR4 adiabatic excitation, 20161.3 Hz spectral width, 8258 complex points.)
Data analysis & statistics
Spectral signals were fit using Bayes (Washington University). To provide higher metabolite signal, the sums of spectra containing hyperpolarized signal from each infusion were used. Metabolite signals were normalized to both the sum of all hyperpolarized signals and to the sum of all metabolite signals as well. Statistical significance was assessed by two-way ANOVA in R with Bonferroni correction to account for multiple comparisons.
Results
In addition to the conversion of pyruvate to lactate, alanine and bicarbonate, aspartate and malate labeled at the 1- and 4-positions were apparent in most experiments, along with [1,4-13C]fumarate adjacent to [1-13C]aspartate (Figure 1). As expected, fasting resulted in lower blood glucose levels (Table 1). The normalized bicarbonate signal was 3.3-fold lower in the fasted rats. By contrast, the aspartate C1 and C4 signals were respectively 2.2- and 3-fold higher with fasting. The trends in metabolite signal were similar when normalized to either total hyperpolarized carbon signal or to the total metabolite signal.After treatment with the PEP-CK inhibitor 3-MPA, the bicarbonate signal was not affected in either the fed or fasted condition (Figure 2A), whereas the [1-13C]aspartate signal was 2.7-fold lower in fed rats and 12-fold lower in fasted following 3-MPA treatment (Figure 2B). Hyperpolarized alanine signals trended lower with fasting and 3-MPA (Figure 2E), while lactate was significantly more prominent after 3-MPA (Figure 2D). Blood lactate was markedly elevated, and glucose lower, in 3-MPA-treated fasted rats (Table 1).
Discussion
The changes with fasting in the conversion of hyperpolarized pyruvate to alanine and bicarbonate in the kidney are similar to those reported previously;4 however, the effect on alanine was not significant here.Bicarbonate production was unaffected by 3-MPA and was significantly lower with fasting, indicating that it results largely from PDH flux (schematic of pathways in Figure 3). This contrasts with what has been observed in the liver, where the bicarbonate signal is significantly reduced in fasted rats following 3-MPA treatment3 and absent in fasted PEP-CK knockout mouse liver.2 On the other hand, the increased aspartate signal with fasting and its decrease with 3-MPA treatment indicate aspartate to be a promising marker of renal gluconeogenic flux. Renal aspartate levels are reportedly increased following 3-MPA treatment,5 so the decreased conversion of pyruvate to aspartate seen here with 3-MPA likely reflects gluconeogenic flux through pyruvate carboxylase and not the metabolite pool size. To the best of our knowledge, this is the first time that the relationship between gluconeogenic metabolism and production of aspartate from hyperpolarized pyruvate in the kidney has been described.
Conclusions
Hyperpolarized pyruvate engages both the glucose-consuming and producing pathways in the rat kidney. Whereas labeled bicarbonate results mainly from PDH flux both in the fed and fasted cases, aspartate produced from hyperpolarized pyruvate is a potential marker of renal gluconeogenic flux.Acknowledgements
The authors gratefully acknowledge Stefan Mitrea, Analina da Silva and Mario Lepore for their assistance with the animal experiments. This work was supported by the Swiss National Science Foundation grants 310030_163050 and PP00P1_157547.References
- Pillot B, Soty M, Gautier-Stein A, Zitoun C, Mithieux G. Protein feeding promotes redistribution of endogenous glucose production to the kidney and potentiates its suppression by insulin. Endocrinology. 2009;150(2):616-624. doi:10.1210/en.2008-0601.
- Merritt ME, Harrison C, Sherry AD, Malloy CR, Burgess SC. Flux through hepatic pyruvate carboxylase and phosphoenolpyruvate carboxykinase detected by hyperpolarized 13C magnetic resonance. Proc Natl Acad Sci USA. 2011;108(47):19084-19089. doi:10.1073/pnas.1111247108.
- Can E, Yoshihara HAI, Bastiaansen JAM, Gruetter R, Comment A. Effects of 3-MPA on in vivo hepatic metabolism of hyperpolarized [1-13C] pyruvate. In: Vol 25. Honolulu; 2017:0166. https://cds.ismrm.org/protected/17MPresentations/abstracts/0166.html.
- Morze von C, Chang G-Y, Larson PEZ, et al. Detection of localized changes in the metabolism of hyperpolarized gluconeogenic precursors 13C-lactate and 13C-pyruvate in kidney and liver. Magn Reson Med. 2017;77(4):1429-1437. doi:10.1002/mrm.26245.
- Vinay P, Coutlée F, Martel P, Lemieux G, Gougoux A. Effect of phosphoenolpyruvate carboxykinase inhibition on renal metabolism of glutamine: in vivo studies in the dog and rat. Can J Biochem. 1980;58(2):103-111. doi:10.1139/o80-015.
Figures
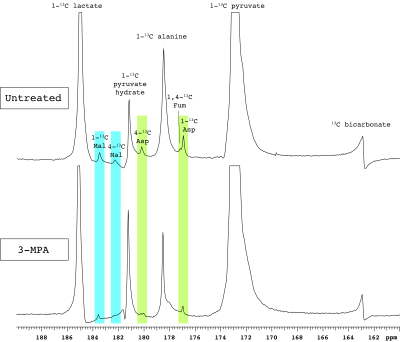
Figure 1. Representative spectra of renal [1-13C]pyruvate metabolism. PEP-CK inhibition by 3-MPA results in markedly lower aspartate and malate signals. Abbreviations: Mal – malate, Asp – aspartate, Fum – fumarate.
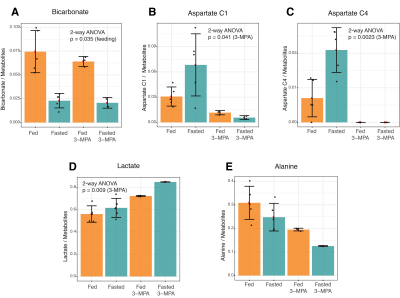
Figure 2. Effects of fasting and 3-MPA treatment on renal metabolism of hyperpolarized 1-13C pyruvate to bicarbonate, aspartate, lactate and alanine. The factors associated with the noted 2-way ANOVA p values are indicated in parentheses.
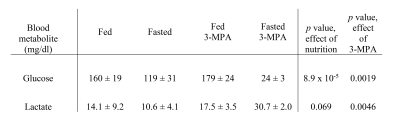
Table 1. Blood glucose and lactate levels (± SD) in fasted and 3-MPA treated rats.
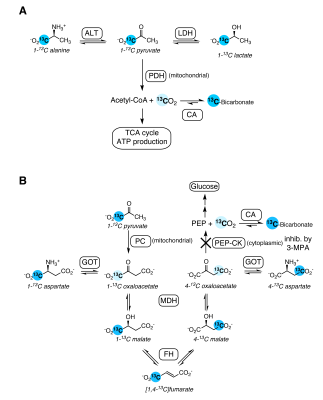
Figure 3. Schematic illustrating the biochemical pathways producing the [1-13C]pyruvate metabolites observed in the kidney. A. Pyruvate oxidation in glycolytic tissue yields labeled 13CO2, detected as bicarbonate. B. Anapleurotic pyruvate metabolism in gluconeogenic tissue also yields bicarbonate, via a 7-step pathway. Labeled intermediates not observed in this study are shown in pale blue.