3588
DNP Polarizing Agents in Preclinical HP MRS: Influence in the Context of Transient Ischemic Stroke1Geneva School of Health Sciences, HES-SO University of Applied Sciences and Arts Western Switzerland, Geneva, Switzerland, 2Laboratory of Functional and Metabolic Imaging, EPFL (Swiss Federal Institute of Technology in Lausanne), Lausanne, Switzerland, 3Department of Clinical Neurosciences, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 4Center for Biomedical Imaging (CIBM), EPFL, Lausanne, Switzerland, 5Image Guided Intervention Laboratory, University of Geneva (UNIGE), Geneva, Switzerland
Synopsis
Used at low concentration in DNP sample preparations, radicals are typically chemically highly reactive species that could potentially interfere with the biochemical processes assessed in HP MR experiments. In this work, we investigate the influence of the nitroxyl radical TEMPOL on the cerebral metabolic response to a bolus of hyperpolarized [1-13C]lactate in a mouse model of transient ischemic stroke. Our results show that TEMPOL, administered at the same dose as when used as a polarizing agent for DNP, alters substantially the metabolic outcome of the experiment and notably results in a significantly different pyruvate labelling after hyperpolarized lactate infusion.
Introduction
Hyperpolarization by dissolution dynamic nuclear polarization1 (dDNP) brought new perspectives for MR molecular imaging. By boosting the sensitivity of metabolites by several orders of magnitude, one can monitor in-vivo biochemical reactions in real-time2.Typically, the DNP sample is a frozen glassy solution containing the metabolite and a small amount of polarizing agent, usually in the form of stable radicals.
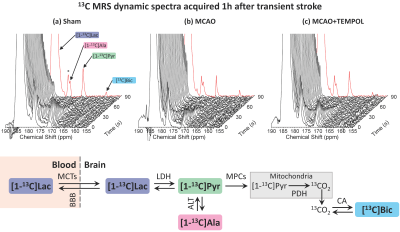
In the context of the development of HP theranostic probes for ischemic stroke, it has been demonstrated that HP lactate provides interesting contrasts3 when administered at a therapeutic dose4,5 after stroke. This initial work employed the widely available and affordable TEMPOL radical as a polarizing agent. The signal of HP lactate was later6 improved by using the more efficient trityl radical benefiting from more effective DNP processes7. Interestingly, by simply replacing the type of polarizing agent, a different trend of labelling of HP [1-13C]pyruvate from [1-13C]lactate was consistently observed in the stroke animals.
The scope of this study is to investigate whether the polarizing agent TEMPOL influences the metabolic outcome of HP [1-13C]lactate bolus in a mouse model of transient ischemic stroke.
Methods
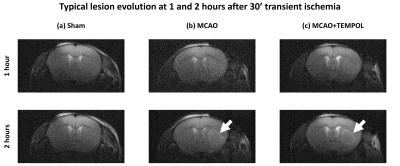
Hyperpolarization: Sodium L-[1-13C]lactate (Sigma-Aldrich) in water/glycerol was doped with 25mM of OX063 radical (Albeda Research) and hyperpolarized in a 7T/1K DNP polarizer8,9 to yield 33.1±8.9% liquid-state polarization at 9.4T prior to injection.Mouse middle cerebral artery occlusion (MCAO) ischemia-reperfusion stroke model: Stroke was induced by transient 30min MCAO in C57BL6/J male mice (6-10 weeks) using the filament technique as previously described10,11. The success of the procedure was assessed by monitoring the regional cerebral blood flow, which must remain below 20% of the initial value during occlusion and increase above 50% of baseline within 10min post-reperfusion. The left femoral vein was cannulated during occlusion for the HP injection. The control group was sham-operated mice without suture insertion or artery ligation.
Acquisition: 13C MRS was performed in a 9.4T/31cm horizontal bore MRI scanner (Varian/Magnex) with a 1H quadrature/13C linear surface coil above the head. At 1h post-reperfusion or post-sham surgery, the sample was dissolved and pushed to a separator/infusion pump8. A therapeutic dose of HP [1-13C]lactate (1.07±0.16µmol/g) was injected into the animal and 13C MRS acquired every 3s with 30° BIR-4 adiabatic pulses. Spatial localization was provided by the coil’s sensitivity profile3. Anatomical T2W images were acquired at 1h and 2h post-reperfusion to verify the evolution of the lesion.
Animal groups: Three groups were scanned: sham-operated mice (n=5), MCAO (n=5), and MCAO+TEMPOL (n=5). In the latter, a dose of 30 μmol/g of 4-Hydroxy-2,2,6,6-tetramethylpiperidin-1-oxyl (TEMPOL) was mixed and injected simultaneously with the HP lactate. The TEMPOL dose was identical as when used as the polarizing agent3.
Data analysis: Spectra acquired in the first 120s post-injection were summed and fitted using the Bayesian Data-Analysis Software Package (Washington University in St. Louis). The normalized metabolites ratios were calculated as previously reported3. Statistical analysis was performed by one-way ANOVA followed by Tukey-Kramer’s test. A p<0.05 was considered significant. All data are presented as means ± SD to correct for multiple comparison.
Results
The MCAO surgery induces a lesion in the left striatum (Fig.1). Cerebral metabolism of HP [1-13C]lactate was observed in healthy and stroke animals, producing [1-13C]pyruvate, [1-13C]alanine and [13C]bicarbonate (Fig.2).The pyruvate-to-lactate ratio (cPLR, Fig.3a) was significantly lower in the MCAO group compared to both sham and MCAO+TEMPOL groups. The cPLR was comparable between sham and MCAO+TEMPOL. The alanine-to-lactate ratio (cALR, Fig.3b) was significantly decreased in the MCAO+TEMPOL group compared to sham, while only a trend could be observed between the MCAO and sham groups. Furthermore, a trend of lower lactate to bicarbonate conversion (cBLR, Fig.3c) was observed after stroke compared to sham.
Discussion
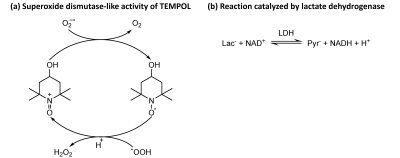
The significantly higher cPLR ratio in the MCAO+TEMPOL group compared to MCAO group indicates that TEMPOL alters the post-ischemic stroke metabolic response to the HP [1-13C]lactate bolus.Despite their low concentration in DNP sample preparations, radicals are typically highly reactive species, which could potentially interfere with the biochemical processes that are being probed. TEMPOL can cross the BBB12 and provides neuroprotection by catalytically decomposing reactive oxygen species produced after stroke13,14 (Fig.4a). This process consumes hydrogen ions, reducing the brain acidity. Consequently, the lactate dehydrogenase equilibrium (Fig.4b) is directed towards higher pyruvate labelling. Since this is a catalytic process, a small TEMPOL dose was sufficient to observe substantial effects. Additionally, the lower acidity should increase the H13CO3/13CO2 ratio, although this parameter was not directly measured in our experiments due to a limited RF pulse bandwidth.
In contrast, trityl radicals are typically more stable15 due to the electron delocalization conferred by their molecular structure. Although they react with the superoxide radical16, the low dosage and non-catalytic reaction are unlikely to substantially affect the experiment outcome. Alanine signals in cerebral MRS experiments rather originate from peripheral tissues than from the brain17–19. It is probable that the change in cALR after stroke is related to muscles around the skull, close to the coil, whose metabolism was affected by the common carotid artery ligature during surgery.
Conclusion
The administration of TEMPOL at the dose commonly used as a polarizing agent for DNP results in a significantly different cerebral metabolic response to HP [1-13C]lactate following ischemic stroke.Acknowledgements
This study is supported by the Swiss National Science Foundation (310030_170155), Biaggi and Juchum Foundations. We thank Drs. Analina Hausin and Stefanita Mitrea for their assistance during the animal experiments, and the Center for Biomedical Imaging of the University of Lausanne, EPFL, University of Geneva, Geneva University Hospitals, Lausanne University Hospital, Leenaards and Louis-Jeantet Foundations.References
1. Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of > 10,000 times in liquid-state NMR. Proc. Natl. Acad. Sci. U. S. A. 100, 10158–63 (2003).
2. Comment, A. & Merritt, M. E. Hyperpolarized magnetic resonance as a sensitive detector of metabolic function. Biochemistry 53, 7333–7357 (2014).
3. Hyacinthe, J.-N. et al. Evaluating the potential of hyperpolarised [1-13C] L-lactate as a neuroprotectant metabolic biosensor for stroke. Sci. Rep. 10, 5507 (2020).
4. Berthet, C. et al. Neuroprotective role of lactate after cerebral ischemia. J. Cereb. Blood Flow Metab. 29, 1780–1789 (2009).
5. Berthet, C., Castillo, X., Magistretti, P. J. & Hirt, L. New evidence of neuroprotection by lactate after transient focal cerebral ischaemia: Extended benefit after intracerebroventricular injection and efficacy of intravenous administration. Cerebrovasc. Dis. (2012) doi:10.1159/000343657.
6. Lê, T. P. et al. Metabolism of the hyperpolarized neuroprotective agents [1-13C] lactate and [1-13C] pyruvate in a mouse model of transient ischemic stroke. in Proc. Intl. Soc. Mag. Reson. Med. 28 0689 (2020).
7. Lumata, L., Merritt, M. E., Malloy, C. R., Sherry, A. D. & Kovacs, Z. Impact of Gd 3+ on DNP of [1- 13C]pyruvate doped with trityl OX063, BDPA, or 4-oxo-TEMPO. J. Phys. Chem. A 116, 5129–5138 (2012).
8. Comment, A. et al. Design and performance of a DNP prepolarizer coupled to a rodent MRI scanner. Concepts Magn. Reson. Part B 31B, 255–269 (2007).
9. Cheng, T., Capozzi, A., Takado, Y., Balzan, R. & Comment, A. Over 35% liquid-state 13C polarization obtained via dissolution dynamic nuclear polarization at 7 T and 1 K using ubiquitous nitroxyl radicals. Phys. Chem. Chem. Phys. 15, 20819–20822 (2013).
10. Castillo, X. et al. A probable dual mode of action for both L-and D-lactate neuroprotection in cerebral ischemia. J. Cereb. Blood Flow Metab. (2015) doi:10.1038/jcbfm.2015.115.
11. Longa, E. Z., Weinstein, P. R., Carlson, S. & Cummins, R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke 20, 84–91 (1989).
12. Zhelev, Z. et al. Nitroxyl radicals for labeling of conventional therapeutics and noninvasive magnetic resonance imaging of their permeability for blood-brain barrier: relationship between structure, blood clearance, and mri signal dynamic in the brain. Mol. Pharm. 6, 504–512 (2009).
13. Rak, R. et al. Neuroprotection by the stable nitroxide Tempol during reperfusion in a rat model of transient focal ischemia. J. Neurosurg. 92, 646–651 (2000).
14. Reddan, J. R. et al. The superoxide dismutase mimic TEMPOL protects cultured rabbit lens epithelial cells from hydrogen peroxide insult. Exp. Eye Res. 56, 543–554 (1993).
15. Ardenkjaer-Larsen, J. H. et al. Agents Intended for Oximetric Imaging. J. Magn. Reson. 133, 1–12 (1998).
16. Rizzi, C. et al. Application of a trityl-based radical probe for measuring superoxide. Free Radic. Biol. Med. 35, 1608–1618 (2003).
17. Miloushev, V. Z. et al. Hyperpolarized 13C pyruvate mouse brain metabolism with absorptive-mode EPSI at 1 T. J. Magn. Reson. 275, 120–126 (2017).
18. Harris, T. et al. Real-time ex-vivo measurement of brain metabolism using hyperpolarized [1-13C]pyruvate. Sci. Rep. 8, 1–14 (2018).
19. Mayer, D. et al. Dynamic and high-resolution metabolic imaging of hyperpolarized [1- 13C]-pyruvate in the rat brain using a high-performance gradient insert. Magn. Reson. Med. 65, 1228–1233 (2011).
Figures