3583
In Vivo Evaluation of Glutaminase Activity with Hyperpolarized [5-13C,4,4-2H2,5-15N]-L-Glutamine in PDAC
Roozbeh Eskandari1, Arsen Mamakhanyan1, Michelle Saoi2, Kristin L Granlund1, Justin Cross2, Craig B Thompson3, and Kayvan Rahimi Keshari1,4
1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Cancer Biology & Genetics Program Share, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Radiology, Memorial Sloan Kettering, New York, NY, United States
1Radiology, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Memorial Sloan Kettering Cancer Center, New York, NY, United States, 3Cancer Biology & Genetics Program Share, Memorial Sloan Kettering Cancer Center, New York, NY, United States, 4Radiology, Memorial Sloan Kettering, New York, NY, United States
Synopsis
Aberrations in glutaminase enzyme expression are associated with a variety of pathologies, and an in vivo probe to quantify flux through this pathway may provide a new layer of information. We developed a custom-synthesized compound, [5-13C,4,4-2H2,5-15N]-L-Glutamine, as a hyperpolarized MRI probe for glutaminase activity. Triple labeling of glutamine and D2O solvation reduces quadrupolar relaxation and extends both T1 and T2, facilitating in vivo imaging. We were able to acquire 13C spectroscopic data on a subcutaneous PDAC xenograft murine model and detect in vivo conversion of hyperpolarized glutamine to glutamate, which permits further exploration of this imaging probe in the future.
Introduction
Glutamine is highly consumed by fast- growing cells, and its main cellular role is providing carbon and nitrogen through various metabolic pathways. One of several enzymes that utilizes glutamine as a substrate is glutaminase, which catalyzes it via aminohydrolysis to glutamate and ammonia. Quantification of glutamine metabolism appears to be crucial since it is involved in multiple diseases or syndromes including cancer and diabetes.1 Due to the global relevance of glutaminase in a variety of diseases and advances in therapeutics related to this pathway, we seek to develop an in vivo metabolic probe. In this study, a xenograft murine model of pancreatic ductal adenocarcinoma (PDAC) as a glutamine-avid tumor has been used. The goal is to develop a probe for real-time measurement of flux in glutamine metabolism and show glutaminase inhibition with CB-839 can be detected with in vivo imaging. Hyperpolarized magnetic resonance provides high transient spin-lattice signal. Fast acquisition sequences in magnetic resonance enable us to obtain dynamics fast biochemical processes. Prior studies using commercial [5-13C]-L-glutamine confirmed that detecting the carbon-5 resonance of [5-13C]-L-glutamate with 13C nuclear magnetic resonance (NMR) could be used to monitor dynamics of the reaction in rat model (Fig.1). However, directly-bonded quadrupolar 14N-nuclei increase signal decay outside the polarizer due to quadrupolar effect at low magnetic field.2 We also learned that T1 can be increased with deuteration of adjacent carbons.3 Here, we provide a modular synthetic strategy for a series of 13C-, 2H(D)-, 15N-isotope-labeled glutamine in high yield. Enriching with 15N decreases low field quadrupolar relaxation. The presence of deuterium next to side chain carbonyl increases T1 by eliminating dipole-dipole relaxation. In addition, we discovered the presence of 15N increases T2.4 Following up our on previous studies, we used D2O phosphate buffer for dissolution resulting in the exchange of protons with deuterium, which increased T1 and T2 relaxations. We have characterized the physical chemical properties of these compounds and have showed triple labeling [5-13C,4,4-2H2,5-15N]-L-Glutamine and dissolving in D2O improved SNR and enabled detection of in vivo glutaminase activity and performed magnetic resonance spectroscopy with two-dimensional resolution.5Methods
Four labeling patterns of glutamine were synthesized using a four-step synthesis (Fig.2B) adapted from previous work on arginine.4 For hyperpolarized experiments, glutamine was polarized in a SPINLab (GE Healthcare) for >2h (5.0T, 0.8K, 139.980GHz). Inversion-recovery and Carr-Purcell-Meiboom-Gill acquisitions for T1 and T2 measurements of the carbon-5 resonance, centered at 178ppm, were performed on a Bruker 14.1T NMR. MIA PaCa-2 cells (ATCC) were cultured in high-glucose DMEM. 5 million cells were xenografted in female athymic nude mouse and imaged when the tumors were approximately 200-300mm3. Hyperpolarized T1 were measured at low field on a 1T 13C-NMR. For in vivo 13C spectroscopy, xenografted mouse was infused with 30 mM [5-13C,4,4-2H2,5-15N]-L-Glutamine via tail vein catheter and imaged on a Bruker 3T MRI following an IACUC-approved protocol. A dual-tuned 1H/13C coil was used to acquire anatomic reference images and spectroscopic data. T2-weighted fast-spin-echo (FSE) images were acquired for anatomic localization, covering the whole tumor (40x40x20-mm FOV, 128x128x10 matrix, TE/TR=69/1800 ms). A sagittal 10mm-slice-selective 30°-excitation was used to acquire 64 spectra (5 kHz bandwidth, 0.1 ms dwell time, 512 points, TE=0.568 ms) with a temporal resolution of 3 s beginning 45 s before dissolution.Results
At 14.1T, the T1 of the glutamine carbon-5 resonance increased with 2H-enrichment: [5-13C]-L-Glutamine T1=10.8±0.55s and [5-13C,4,4-2H2]-L-Glutamine T1=12.74±0.69s. 15N-enrichment provided minimal increase: [5-13C,5-15N]-L-Glutamine T1=10.9±0.5s. Triple-labeling resulted in the largest increase: [5-13C,4,4-2H2,5-15N]-L-Glutamine T1=12.74±0.69s) (Fig.2B). Dissolution in D2O increased T1 in all species, e.g., [5-13C,4,4-2H2,5-15N]-L-Glutamine T1=15.58±0.7s. Hyperpolarization decay at 1T showed similar trends: [5-13C,4,4-2H2,5-15N]-L-Glutamine relaxation in phosphate buffer in D2O decreased ~3.3-fold resulting in a T1=72±5s. (Fig.3C) Interestingly, by using slightly acidic phosphate buffer (pH= 3.1), accounting for the NaOH in the prep formulation, no side production of pyroglutamate or glutamate were detected in the final dissolution as highlighted in the hyperpolarized 13C dynamic decay (Fig.3D). At 14.1T, the T2 of the carbon-5 resonance increased with 15N-enrichment: [5-13C]-L-Glutamine T2=0.474±0.029s and [5-13C, 5-15N]-L-Glutamine T2=4.82±0.23s. 2H-enrichment provided a minimal increase: [5-13C,4,4-2H2]-L-Glutamine T2=0.521±0.048s. Triple labeling provided the largest increase: [5-13C,4,4-2H2,5-15N]-L-Glutamine T2=7.28±0.48s. Dissolution in D2O increased the T2 in all species, e.g., [5-13C,4,4-2H2,5-15N]-L-Glutamine T2=12.23±0.36s, ~26-fold enhancement (Fig.3B). Sagittal slab dynamic 13C HP MRS in a mouse injected with hyperpolarized [5-13C,4,4-2H2,5-15N]-L-Glutamine revealed in vivo conversion to hyperpolarized glutamate (Fig. 4A,B). The treatment with glutaminase inhibitor (CB-839) (single dose, 200mg/kg oral gavage) shows almost no conversion to glutamate (Fig. 4C).Discussion
2H-enricment has a substantial impact on extending glutamine carbon-5 resonance T1 at high or low field. 15N-enrichment has an insignificant effect on thermal T1, but reduces quadrupolar relaxation and alleviates rapid depolarization following dissolution when the sample is removed from a strong magnetic field. 15N-enrichment increased T2 at all field strengths, thereby improving imaging quality. Conversion of multi-labeled hyperpolarized glutamine can be used for glutaminase activity in vivo. 13C spectroscopic data in a xenograft model with hyperpolarized glutamine demonstrated detection of higher metabolic flux in the tumor as compared to glutaminase inhibitor treatment, suggesting delivery and metabolic conversion occur rapidly and further hyperpolarized studies should be pursued.Acknowledgements
This work was supported by NIH/NCI Cancer Center Support Grant P30 CA008748, NIH S10 OD016422 as well as Tow Foundation, Thompson Family Foundation.References
1. Pavlova, N.N, et al. 2016. The Emerging Hallmarks of Cancer Metabolism. Cell Metab, 23. p.27-47.2. Cabella, C, et al, 2013. In vivo and in vitro liver cancer metabolism observed with hyperpolarized [5-13C]glutamine. J Magn Reson, 232. p.45-523. Qu, W., et al. 2011. Facile synthesis [5-13C-4-2H2]-L-glutamine for hyperpolarized MRS imaging of cancer cell metabolism. Acad Radiol,18,9. p932-9.4. Cho, A. et al. 2019. Hyperpolarized [6-13C,15N3]-Arginine as a Probe for in Vivo Arginase Activity. ACS Chem Biol, 14,4. p.665-6735. Cho, A. et al. 2018. A non-synthetic approach to extending the lifetime of hyperpolarized molecules using D2O solvation. J Magn Reson, 295.p.57-62.Figures
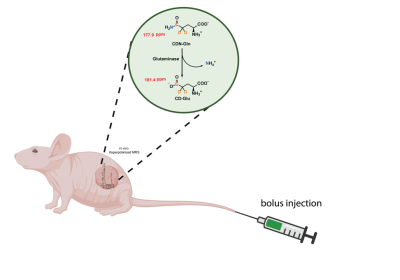
Conversion of [5-13C,4-2H2,5-15N]-L-Glutamine to [5-13C,4-2H2]-L-Glutamate with glutaminase in tumor. Red, orange, blue represent sites of 13C, 2H and 15N enrichment, respectively.
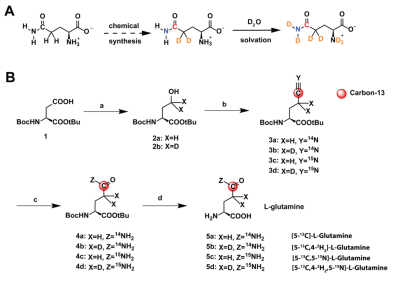
A Glutamine probe design: 13C, 2H and 15N enrichment through chemical synthesis and subsequent D2O solvation during dissolution in [5-13C,4-2H2,5-15N]-L-Glutamine B Synthetic scheme for four different labeling patterns of glutamine a) i. ClCOOEt, NEt3, THF, -5 oC, 30 min, ii. NaBH4(D4), H2O (D2O), 0 oC, 1 h b) [13C,14N(15N)]-KCN,18-Crown-6, P(n-Bu)3, CCl4, CH3CN, rt, 24h c) acetaldoxime, RhCl(PPh3)3 (0.5 mol%), toluene, 110 oC, 24h d) TFA/ DCM (1:1), rt, 12h
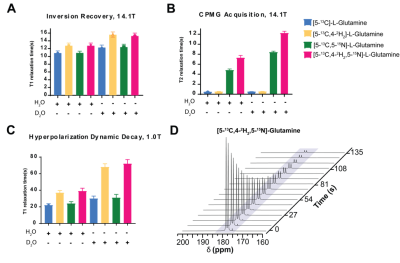
A Four different labeling patterns of glutamine (30mM) carbon-5 resonance T1 measured at 14.1T in H2O or D2O, 200 mM phosphate at pH 7.4 B Four different labeling patterns of glutamine (30mM) carbon-5 resonance T2 measured at 14.1T in H2O or D2O, 200 mM phosphate at pH 7.4 C Hyperpolarization decay of four different labeling patterns of glutamine (30mM) carbon-5 resonance T1 measured at 1.0T in H2O or D2O, D Hyperpolarization decay of [5-13C,4-2H2,5-15N]-L-Glutamine in 200 mM phosphate buffer in D2O acquired with a 10° flip angle every 3s (every third spectra is shown).
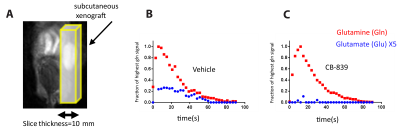
A T2-weighted 1H MRI of mouse, with tumor (right) highlighted as regions of sagittal slab B Dynamic of conversion of glutamine to glutamate in the tumor with vehicle. C Dynamic of conversion of glutamine to glutamate in the tumor with glutaminase inhibitor CB-839