3582
Off-Resonance Correction with Self-Estimated Field Map for Hyperpolarized 13C Metabolic Imaging1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2HeartVista, Inc., Los Altos, CA, United States, 3GE Healthcare, Sunnyvale, CA, United States
Synopsis
Hyperpolarized 13C magnetic resonance imaging is a non-invasive imaging tool to assess metabolic process in-vivo that has been most commonly applied to imaging cancer and heart disease. Spiral readout, as a rapid acquisition technique, is commonly used to acquire hyperpolarized metabolic data. However, the spiral readout is sensitive to the off-resonance effect and has blurring artifacts as a result. The blurring also affects the measurement of metabolism. In this project, we investigated two off-resonance correction methods with self-estimated field maps and applied them on different anatomies.
Introduction
Hyperpolarized 13C magnetic resonance imaging (MRI) is a non-invasive imaging tool for assessing metabolism, including tumor lactate production1,2 as well as metabolism profiles in the brain3,4 and heart5,6. Considering the finite decay and non-renewable magnetization generated by hyperpolarization, the single-shot spiral-out readout combined with spectral-spatial RF excitations is a common efficient technique to encode the k-space data7. However, the spiral readout is sensitive to B0 inhomogeneity, which results in image blurring8. Off-resonance correction methods have been applied in prior hyperpolarized 13C studies using spiral readouts, including 1)a multi-frequency automatic field map estimation scheme8,9; 2)a model-based reconstruction to estimate the image and field map alternatively10. In this work, we improved the current off-resonance correction methods to self-estimate the field map and compared the performance in HP 13C studies in the different anatomies.Methods
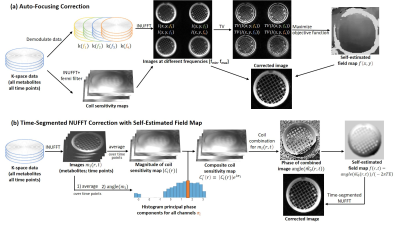
Auto-Focusing CorrectionWe modified the auto-focusing method with spiral readout for proton MRI8, and adapted this method to Hyperpolarized 13C data. The flowchart is shown in Figure 1(a). To begin with, the coil sensitivity maps were measured by smoothing the reconstructed pyruvate images. And the k-space data were demodulated at several frequencies with a suitable range to cover the estimated B0 inhomogeneity map. The field map can be estimated by maximizing the demodulated images ($$$I(x,y,f)$$$) with a total variation (TV) as the criteria, as shown below:$$
f(x,y)=\underset{f}{\mathrm{argmax}}\int\int_{A(x,y)}^{}{TV(I(x',y',f))dx'dy'}
$$Here, $$$A(x,y)$$$ is a summation window function at the location $$$(x,y)$$$. With the estimated field map, the corrected image was pieced together pixel by pixel from demodulated images ($$$I(x,y,f)$$$).
Time-Segmented NUFFT Correction with Self-Estimated Field Map
Considering the spiral readout, the image ($$$m_{i}(r,t)$$$) at $$$t$$$ time frame for $$$i$$$-th channel can be represented as $$
m_i(r,t)=m_0(r,t)C_i(r)e^{(-i2\pi f(r,t)TE)}
$$Where $$$C_i(r)$$$ is the coil sensitivity map of $$$i$$$-th channel, $$$f(r,t)$$$ is the off-resonance frequency. Estimation of field map is illustrated in Figure 1(b). Each channel image ($$$m_i(r,t)$$$) of all metabolites at all time points were reconstructed with NUFFT11, then were averaged over time points. We estimated the phase produced by the coil by finding the phase ($$$\sigma_i$$$) with highest value from the histogram of averaged images. The magnitude ($$$\mid C_i(r) \mid$$$) of coil sensitivity map was extracted and then merged into the composite coil sensitivity map ($$$C_i^*(r)$$$) with the relationship$$
C_i^* (r)=|C_i (r)| e^{iσ_i}
$$The composite images ($$$\hat{m_0}(r,t)$$$) were then derived from the composite coil sensitivity map to estimate the field map by$$
f(r,t)=angle(\hat{m_0}(r,t))/(-2{\pi}TE)
$$Finally, corrected images were reconstructed with time-segmented NUFFT12.
Phantom Experiments
Ethylene glycol (EG) phantom data were acquired on a 3T GE scanner using multi-slice 2D single-shot spiral readout to test the two self-estimated off-resonance correction methods. Data were acquired with a clamshell transmit coil and an 8-channel paddle receive array coil. Scan parameters were FOV=58X58cm2, resolution=10.58X10.58mm2, slice thickness=100mm, TE/TR=10/500ms, readout time=22ms, NEX=2400.
In-Vivo Experiments
We compared the two methods on different in-vivo anatomies: brain, heart, kidney. All in-vivo experiments were acquired on the 3T GE scanner with the same setting as the phantom experiment. In the in-vivo test, slice thickness=21mm, NEX=30. Other scan parameters were
1) brain experiments FOV = 39X39cm2, resolution = 15X15mm2, TE/TR = 10/125ms.
2) cardiac experiments FOV = 30X30cm2, resolution = 12X12mm2, TE/TR = 10/53ms.
3) renal experiments FOV = 75X75cm2, resolution = 15X15mm2, TE/TR = 10/100ms.
Results
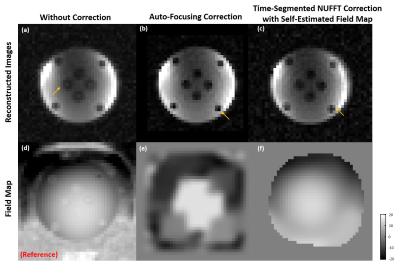
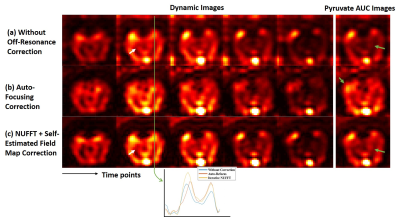
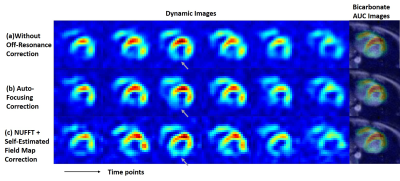
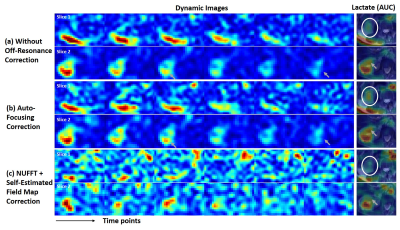
Figure 2 shows reconstructed images of EG phantom. Figure 3-5 show reconstruction results 13C pyruvate brain images, bicarbonate cardiac images and lactate renal images, respectively. Without off-resonance correction, the images have blurring at the edge between different materials, shown in Figure 2(a). In Figure 2, the auto-focusing correction method shows a better apparent correction performance with a sharper edge, indicated by the yellow arrows. However, the autofocus estimated field map is very different from the acquired field map while the time-segmented appears more accurate. In Figure 5, the auto-focusing correction method also shows a better correction performance with an intensity increase than the image without off-resonance correction, indicated by the white circles and grey arrows. In Figure 3&4, the self-estimated field map and time-segmented NUFFT correction method shows a better correction performance. In Figure 3, we chose a line profile to compare the intensity of three methods. The results of using the iterative NUFFT correction method has an improvement of intensity. In Figure 4, only using the method(c) provides visualize of the entire left ventricle myocardium, shown by the grey arrows.Discussion
After comparing the results of two correction methods applying to different anatomies, the two methods are suitable for different regions. Considering the time-segmented NUFFT method may amplify the noise during iterations, this method is suitable for high SNR images. At the same time, the self-estimated field map is based on the assumption that the coil applies a constant phase on the image, so the estimated field map may have an offset frequency. The auto-focusing method uses a TV criteria to find the sharpest image, so this method is more suitable to clear structure images. And because the corrected image is pieced together, the SNR of corrected image should be same as the image reconstructed by the conjugate phase. In conclusion, the off-resonance effect in Hyperpolarized 13C data with spiral readout can be reduced by the self-estimated B0 inhomogeneity.Acknowledgements
This work is supported by the National Institute of Biomedical Imaging and Bioengineering (P41EB013598, U01EB026412), Myokardia Myoseeds award, UCSF Resource Allocation Program, and American Cancer Society Research Scholar Grant 131715-RSG-18-005-01-CCE.References
[1] Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK. Rising incidence of small renal masses: a need to reassess treatment effect. J Natl Cancer Inst. 2006; 98(18): 1331-1334.
[2] Welch HG, Skinner JS, Schroeck FR, Zhou W, Black WC. Regional Variation of Computed Tomographic Imaging in the United States and the Risk of Nephrectomy. JAMA Intern Med. 2018;178(2):221-227.
[3] Mayer, Dirk, et al. “Dynamic and high-resolution metabolic imaging of hyperpolarized [1-13C]-pyruvate in the rat brain using a high-performance gradient insert.” Magnetic resonance in medicine 65.5 (2011): 1228-1233.
[4] Josan, Sonal, et al. “Effects of isoflurane anesthesia on hyperpolarized 13C metabolic measurements in rat brain.” Magnetic resonance in medicine 70.4 (2013): 1117-1124.
[5] Cunningham, Charles H., et al. "Hyperpolarized 13C metabolic MRI of the human heart: initial experience." Circulation research 119.11 (2016): 1177-1182.
[6] Rider, Oliver J., et al. "Noninvasive in vivo assessment of cardiac metabolism in the healthy and diabetic human heart using hyperpolarized 13C MRI." Circulation Research 126.6 (2020): 725-736.
[7]Tyler, Andrew, et al. “A 3D hybrid-shot spiral sequence for hyperpolarized imaging. “ Magnetic Resonance in Medicine 85.2 (2020): 790-801.
[8] Man, Lai‐Chee, John M. Pauly, and Albert Macovski. “Improved automatic off‐resonance correction without a field map in spiral imaging.” Magnetic resonance in medicine 37.6 (1997): 906-913.
[9] Lau, Angus. Frequency-selective Methods for Hyperpolarized 13C Cardiac Magnetic Resonance Imaging. Diss. 2012.
[10] Julia Traechtler, et al. “Joint Image and Field Map Estimation for Multi-Echo Hyperpolarized 13C Metabolic Imaging.” ISMRM 2020, abstract ID: 3016.
[11] Fessler, Jeffrey A., and Bradley P. Sutton. “Nonuniform fast Fourier transforms using min-max interpolation.” IEEE transactions on signal processing 51.2 (2003): 560-574.
[12] Sutton, Bradley P., Douglas C. Noll, and Jeffrey A. Fessler. “Fast, iterative image reconstruction for MRI in the presence of field inhomogeneities.” IEEE transactions on medical imaging 22.2 (2003): 178-188.
Figures