3579
Hyperpolarized 13C MRI of Fumarate Metabolism for Imaging Necrosis in Hepatitis Mice by Parahydrogen-induced Polarization.
Shingo Matsumoto1, Neil J. Stewart1, Hitomi Nakano1, Takuya Hashimoto2, and Hiroshi Hirata1
1Information Science and Technologies, Hokkaido University, Sapporo, Japan, 2Department of Chemistry, Chiba University, Chiba, Japan
1Information Science and Technologies, Hokkaido University, Sapporo, Japan, 2Department of Chemistry, Chiba University, Chiba, Japan
Synopsis
Hyperpolarized [1-13C]fumarate is a promising MRI biomarker for cellular necrosis, which plays an important role in various diseases and cancer treatment response. To demonstrate the feasibility of hyperpolarized MRI of [1-13C]fumarate metabolism using parahydrogen-induced polarization (PHIP) – a low-cost alternative to dissolution dynamic nuclear polarization (dDNP), a cost-effective synthetic pathway for high-yield production of a fumarate precursor [1-13C]acetylenedicarboxylate was developed. A simple hydrogenative PHIP set-up was used to generate hyperpolarized [1-13C]fumarate at sufficient polarization and concentration for in vivo [1-13C]fumarate metabolic MRS/MRI in acetaminophen-induced murine model of hepatitis.
INTRODUCTION
Cell death is commonly observed in various diseases including inflammation, cardiac infarction, stroke, cancer treatment etc. Metabolic flux of exogenously administered hyperpolarized 13C fumarate, prepared by dissolution dynamic nuclear polarization (dDNP), into malate can serve as a non-invasive imaging biomarker for necrotic cell death.1 Parahydrogen-induced polarization (PHIP) is a low-cost alternative to dDNP.2 Although typical parahydrogenation of alkynes result in cis-alkenes as reaction products, a novel trans-selective ruthenium-based catalyst has recently been shown to demonstrate hyperpolarized [1-13C]fumarate by parahydrogen addition to [1-13C]acetylene dicarboxylate.3,4 In this study, we report 1) a cost-effective new synthetic route for high yield of the fumarate precursor [1-13C]acetylene dicarboxylate, 2) comparison of available 13C polarization by several different 1H-to-13C spin order transfer methods including a magnetic field cycling (MFC) and INEPT-type pulse sequences L-PH-INEPT+ and S2hM to generate hyperpolarized [1-13C]fumarate, and at the first time 3) the feasibility of hyperpolarized 13C MRI of fumarate metabolism in a murine acetaminophen-induced model of hepatitis using PHIP.METHODS
A non-gaseous [1-13C]sodium acetate was used as a starting material to synthesize fumarate precursor [1-13C]acetylene dicarboxylate (Figure 1a). Parahydrogen gas was generated using a home-built system, which reproducibly yields a parahydrogen concentration of 90—95% with a lifetime of the order of weeks in the storage cylinders, as quantified by a Raman spectrometer. Immediate after 30 sec parahydrogen (>90% purity, 6-8 bar) bubbling of a mixture of [1-13C]acetylene dicarboxylate and Cp*Ru(MeCN)3PF6 as a trans-selective hydrogenation catalyst, either of three different spin order transfer methods; MFC, L-PH-INEPT+ or S2hM was applied. All anatomic and hyperpolarized 13C MRS/MRI studies were conducted by using our lab-made MRI system with a 1.5T permanent magnet and a dedicated multinuclear spectrometer (Japan REDOX, Fukuoka, Japan). Hyperpolarized 13C MRI measurements of fumarate metabolism in hepatitis model mice were conducted 4-6 hours after intraperitoneal injection of acetaminophen.RESULTS
The total yield of the final product [1-13C]acetylenedicarboxylate by the developed new synthesis pathway with [1-13C]sodium acetate as a starting material was 43%. More than 12% 13C polarization at the time of MRI measurement was achieved by MFC and S2hM as 1H-to-13C spin order transfer methods after parahydrogenation reaction (Figure 1b). Mixing the hyperpolarized [1-13C]fumarate with liver homogenate showed time dependent generation of cell death biomarker hyperpolarized [1-13C]&[4-13C]malate peaks (Figure 3). Non-invasive 2D chemical shift imaging (CSI) of hyperpolarized [1-13C]fumarate metabolism showed strong peaks of hyperpolarized [1-13C]&[4-13C]malate around the liver region in a hepatitis mouse 4-6 hours after injection of acetaminophen.DISCUSSION
In this study the PHIP procedure including hydrogenation and removal of Ru catalyst by a metal trapping column filling a QuadraPure TU is conducted by hand, resulting in a significant loss of polarization. We are currently developing a fully automated PHIP polarizer system and such automation of the PHIP procedure should improve the available 13C polarization of metabolic tracer, and so the quality of the image. Although we did not see any cytotoxic effects of unreacted acetylenedicarboxylate remained in the injection solution, complete removal of the reaction substrate is desirable for future studies.CONCLUSION
We have demonstrated initial feasibility of in vivo MRI of PHIP-polarized [1-13C]fumarate metabolism for detection of cell necrosis in a mouse model of hepatitis. These findings should facilitate further pre-clinical studies and represent key steps in the eventual translation to clinical applications using the low-cost and rapid polarization time (<1 min) PHIP polarizer.Acknowledgements
This research was supported by AMED (grant no. JP20hm0102061) and the Japan Society for the Promotion of Science (JSPS) KAKENHI (grant no: 18K19385). NJS was supported by a postdoctoral research fellowship from JSPS.References
- Gallagher FA, Kettunen MI, Hu DE et al. Production of hyperpolarized [1,4-13C2]malate from [1,4-13C2]fumarate is a marker of cell necrosis and treatment response in tumors. Proc Natl Acad Sci USA. 2009;106(47):19801-19806.
- Stewart NJ, Matsumoto S. Biomedical Applications of the Dynamic Nuclear Polarization and Parahydrogen Induced Polarization Techniques for Hyperpolarized 13C MR Imaging. Magn Reson Med Sci. 2019.
- Ripka B, Eills J, Kouřilová H et al. Hyperpolarized fumarate via parahydrogen. Chem Commun. 2018;54(86):12246-12249.
- Eills J, Cavallari E, Carrera C et al. Real-time nuclear magnetic resonance detection of fumarase activity using parahydrogen-hyperpolarized [1-13C]fumarate. J Am Chem Soc. 2019;141(51):20209-20214.
Figures
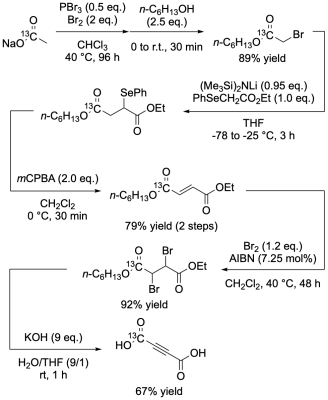
Figure 1. Scheme of a cost-effective synthesis of
fumarate precursor [1-13C]acetylenedicarboxylic
acid from [1-13C]sodium acetate as a starting material
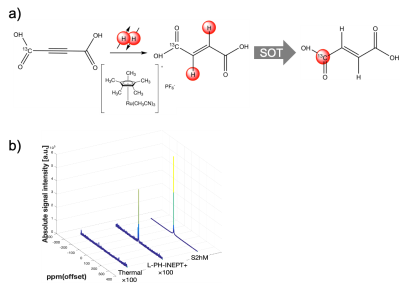
Figure 2. (a)
Preparation
of hyperpolarized [1-13C]fumarate
by trans-alkenylation
with parahydrogen.
(b) 13C
NMR of hyperpolarized [1-13C]
fumarate using different 1H-to-13C spin order transfer pulse sequences.
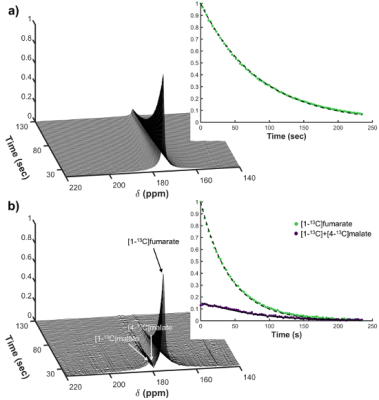
Figure 3. (a)
Dynamic 13C NMR
spectra of hyperpolarized [1-13C]fumarate
at 176 ppm produced by MFC-based PHIP. (b) Generation of hyperpolarized [1-13C]
& [4-13C]malate
at around 181 ppm by mixing hyperpolarized [1-13C]fumarate
with mouse liver homogenates.
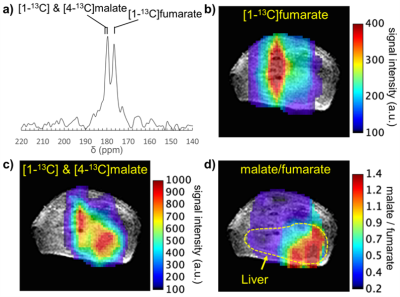
Figure 4. In
vivo
CSI of hyperpolarized [1-13C]fumarate
metabolism in an acetaminophen-induced hepatitis mouse. (a) Representative 13C NMR
spectrum of hyperpolarized [1-13C]fumarate
and its metabolite at the liver. (b) map of hyperpolarized [1-13C]fumarate
CSI signal intensity overlaid on an
anatomical 1H MRI
image. (c) map of hyperpolarized [1-13C]
& [4-13C]malate.
(d) parametric map of the malate/fumarate ratio; a biomarker of cellular
necrosis.