3572
Performance of XTC imaging in a free-breathing mouse model exploring variable saturation delay times1Radiology, University of Pennsylvania, Philadelphia, PA, United States, 2University of Pennsylvania, Philadelphia, PA, United States
Synopsis
XTC imaging takes advantage of the exchange between gas and dissolved phase Xe to provide information on lung function, as direct dissolved phase imaging is limited in signal. In this study, we demonstrate XTC imaging in a regular and transgenic mouse model. We aim to optimize depolarization effectiveness by varying delay time and number of saturation pulses applied in the imaging scheme and demonstrate the dependence of depolarization to these parameters.
Introduction
Hyperpolarized Xe-129 (HXe) MR imaging is a powerful tool for evaluating lung function and structure. The effectiveness of directly imaging dissolved-phase (DP) Xe is limited, owever, as only 2% of inhaled Xe dissolves in lung parenchyma and red blood cells (RBC). To address this issue, Xenon-polarization Transfer Constant (XTC) imaging is used to extract information about dissolved-phase components of Xe from the more plentiful gas-phase (GP) signal. In XTC imaging, Xe signal is saturated at the DP resonance and the resulting depolarization in GP signal reflects the amount of DP Xe and its rate of exchange (1).In this work, we utilize XTC imaging in free-breathing wild-type and transgenic mice in order to better mimic normal physiological conditions as well as to allow for longitudinal imaging (2). To ensure that the results properly reflect GP/DP exchange, images must exhibit significant contrast between pre- and post-saturation schemes. We therefore optimized our imaging scheme by applying various combinations of delay time and number of pulses.
Methods:
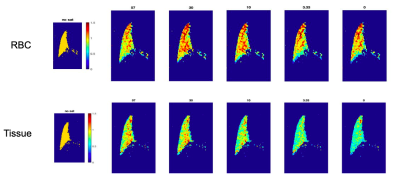
Imaging was performed in one wild-type mouse (C59BJ6) (25 g) and one transgenic mouse (B6;129-Hbatm1(HBA)Tow Hbbtm2(HBG1,HBB*)Tow/Hbbtm3(HBG1,HBB)Tow/J, Jackson Laboratory) (20 g). Wild-type mice exhibit only one peak for dissolved-phase Xe; the transgenic model, which is homozygous for human hemoglobin, exhibits two peaks: for Xe dissolved in the lung parenchyma and in red blood cells (RBC), at 199 ppm and 217 ppm, respectively (3). Enriched 129Xe gas was polarized using a prototype commercial optical pumping system (Xemed, NH) and dispensed into a 0.75 L Tedlar bag within a pressurizable chamber. Animals were secured into a bed with a nose cone, into which Xe gas was delivered continuously at ~4 psi. An exhaust of ~100 mL/min exhaled gas was drawn out from the nose cone, prompting the mouse to inhale the delivered Xe and oxygen from the surrounding air. All imaging was performed on a 9.4 T scanner (Bruker Inc, MA).XTC images were acquired using a 2D FLASH sequence, triggered at every breath to correspond with a different phase-encode line. Frequency-selective saturation pulses were implemented into the sequence to precede each acquisition. In wild type mice, this saturation pulse was centered at the dissolved peak (195* ppm) with a FWHM of 2350 Hz (gaussian, 2.7 ms, 180 deg). Various delay time/# of pulse combinations were applied, encompassing the same total saturation time of 200 ms and ranging from 4 ms/50 pulses to 66.7 ms/3 pulses. Each set of parameters was acquired twice, using an abccba acquisition scheme, to average and account for T1-influenced signal loss. In transgenic mice, these datasets were acquired at two different saturation pulses, centered at the RBC and tissue peaks, 217 ppm and 199 ppm, respectively (gaussian, 10 ms, 180 deg; excitation FA 90, TR/TE = 2/40 ms, matrix size = 64x64, FOV = 20 mm). Because the saturation pulse in this scheme was longer to allow for selectivity around each peak, pulse combinations were limited to a range between 20 pulses/10 ms and 3 pulses/67 ms. Depolarization ratio maps were calculated as the ratio of these images to the control images acquired with 0 saturation pulses.
Results/Discussion
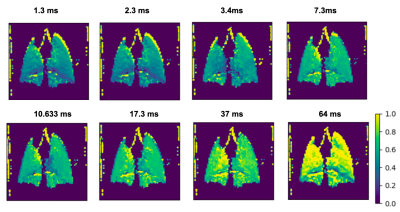
By applying the saturation scheme using a variety of different parameters, we aimed to identify the optimal conditions for achieving sufficient contrast using XTC imaging in the mouse model. By balancing the number and duration of pulses, it should be possible to saturate the dissolved phase resonances more dramatically, causing more depolarization in the gas phase peak. However, longer delay times may require reducing the number of pulses, thereby allowing more time for exchange between GP and DP—another critical component in producing sufficient contrast between pre/post saturation GP signals. Figure 1 shows depolarization maps for a range of saturation delay times(D) and number of pulses (N) in a wild type mouse. Here, the imaging scheme produces a depolarization of about 30% when N >= 30, with minimal differences between 30-50. This suggests that a delay time of ~4 ms is sufficient to allow for exchange between GP and DP Xe, and that 30 pulses produce enough contrast to obtain an accurate DP/GP ratio. From these ratios, the depolarization per pulse at each pixel was calculated using the equation: (1 - S1/S0)1/N, where S1 represents the signal of the image with saturation, S0 of the image without, and N the number of saturation pulses. In Figure 2, depolarization per pulse is plotted across a range of delay times for dissolved phase saturation in wild-type mice and both RBC and tissue saturation in transgenic mice, displaying a trend of increasing depolarization per pulse with increasing delay time. The mean depolarization in the lungs at each delay time does not follow the same trend, as the total depolarization is also influenced by the number of pulses applied. Figure 3 displays loss per pulse across number of pulses in each model, confirming an inverse relationship with increasing number of pulses.Conclusion
In this study, we demonstrated the feasibility of XTC imaging in wildtype and transgenic free-breathing mice and explored the relationship between delay time and number of saturation pulses for optimizing the depolarization of GP Xe.Acknowledgements
No acknowledgement found.References
[1] Ruppert, Kai, et al (2000). Probing lung physiology with xenon polarization transfer contrast (XTC). MRM 44(3), 349-357.
[2] Loza, Luis, et al (2019). Quantification of Ventilation and Gas Uptake in Free-Breathing Mice with Hyperpolarized 129MRI. IEEE 38(9), 2081-2091.
[3] Freeman, Michael, et al (2013). Enabling Hyperpolarized 129Xe MR Spectroscopy and Imaging of Pulmonary Gas Transfer to the Red Blood Cells in Transgenic Mice Expressing Human Hemoglobin. Magna Res Med 70(9), 1192-1199.
Figures
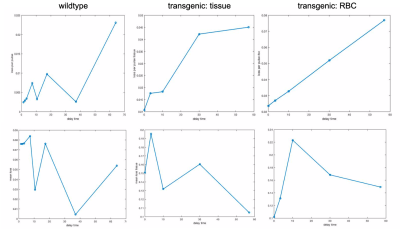
Top: plots representing signal loss per pulse in the gas peak at a range of delay times, in a wild type mouse (saturated at dissolved), and in a transgenic mouse (saturated at tissue and RBC).
Bottom plots representing mean depolarization at each delay time wild type and transgenic models.