3567
3D isotropic spectroscopic imaging of hyperpolarized 129Xe in the human brain1University of Sheffield, Sheffield, United Kingdom, 2GE Healthcare, Munich, Germany
Synopsis
3D isotropic images of hyperpolarized 129Xe dissolved in the brain were acquired with 1 L of inhaled xenon gas dose using a density-weighted MR spectroscopic imaging method. Images were acquired from 2 healthy male volunteers. The data was acquired with a voxel size of (2cm)3 and reconstructed to a voxel size of (0.625 cm)3 through zero filling. The isotropic voxel size and the high spectral resolution surpass previous reports using conventional 2D and echo planar spectroscopic imaging methods.
Introduction
MR imaging of hyperpolarized (HP) 129Xe dissolved in the human brain has recently been demonstrated (1), where the images obtained are weighted towards uptake of xenon gas by the grey matter (1). These images were acquired at the concentration achieved with inhaled xenon gas dose of 1 L. Recently, assessing the feasibility of using hyperpolarized 129Xe for clinical applications such as stroke (2), Alzheimer’s (3) and functional MRI (4) has been reported. 129Xe dissolved in the brain exhibits unique chemical shifts for various biochemical compartments (5) which can enable the estimation of gas transfer rate between the compartments (6), thus providing additional physiological information.Most spectroscopic studies to date have employed whole-brain spectroscopy or full plane projection for chemical shift imaging (5,7), and most imaging methods using spatial encoding have employed a very thick slice of 50 mm or more (1-4). Thus, a key aspect that is limiting the research and development of wide range of MR methods for HP 129Xe dissolved in the brain is the image resolution. The purpose of this study was to investigate methods to achieve higher spectroscopic imaging resolution in both the spatial and spectral domains, by using 3D density-weighted MR spectroscopic imaging methods (8).
Methods
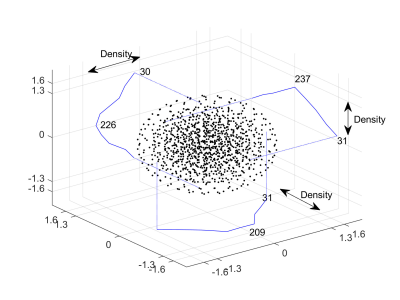
HP 129Xe brain MRI was performed on 2 healthy male volunteers (age 37 and 38) on a 1.5 T GE HDx scanner, with informed written consent. The xenon gas dose for each of the imaging sessions was around 1 L. 129Xe was polarized to > 30% polarization using a regulatory approved spin exchange optical pumping polarizer (POLARIS, Sheffield, UK) (9). MR imaging was performed with a home built 4 channel receiver RF coil (1).The k-space trajectory of the spectroscopic imaging sequence is shown in Figure 1 (8), which indicates that the density at the center of k-space is 8 times higher than at the edge of k-space, imposing a spatial apodization during acquisition. Hence avoiding the SNR loss as compared to apodizing during reconstruction. A 3D matrix of 103 corresponds to a total of 1101 RF acquisitions. A rectangular non-spatially selective RF pulse of 144 µs led to partial suppression of the remaining gas signal leaking in from the head. Imaging parameters were: flip angle = 5.5°, receiver bandwidth = 10 kHz with 288 number of points, field of view = 200 x 200 x 200 mm3, acquisition matrix of 10 x 10 x 10 implies acquired voxel size of 2 x 2 x 2 cm3 and repetition time = 31.2 ms. Total scan duration was 25 s. The density-weighted spectra were reconstructed spatially onto a Cartesian grid and interpolated spatially to 32 x 32 x 32 matrix by zero-filling. The spectral peak corresponding to HP 129Xe resonance in grey matter was used to generate images in all three planes.
Results
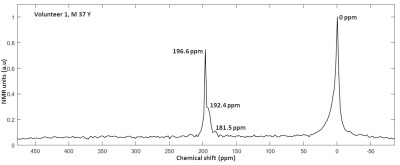
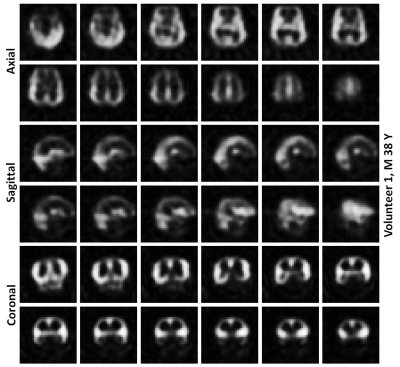
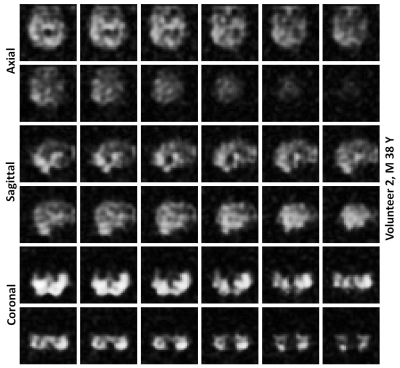
Average spectrum of all the spatially resolved 1101 RF-acquisitions from volunteer 1 is shown in Figure 2. Average spectrum indicates 3 spectral peaks from dissolved-phase 129Xe in the head. Images of HP 129Xe xenon dissolved in the grey matter at the acquired slice thickness of 2 cm are shown for both volunteer in Figure 3. Images interpolated to a matrix size of 32 x 32 x 32 for both volunteers are shown in Figures 4 and 5. The interpolated slice thickness in the images in Figure 4 and 5 is 0.625 cm.Discussion
Observable dissolved-phase spectral peaks in the head have been previously assigned to grey matter (196.6 ppm), white matter (192.4) and soft muscular tissue outside the brain (181.5) (5). Spectral peak at 0 ppm is from 129Xe in gas-phase in air spaces in the head such as trachea or nasal cavity.With 3D density-weighted MR spectroscopic imaging, the density of NMR spectral acquisitions in the k-space is weighted towards the centre, which has the benefit of boosting the sensitivity of HP 129Xe dissolved in the brain. The trade off with this k-space filter is that the higher frequency regions near the edge of k-space, which primarily contributes to the structural details in the image, are smoothed. The k-space trajectory and associated imaging parameters TR and flip angle, when combined with the arterial blood input function, T1 of xenon in the compartments of the brain and the breathing manoeuvre define quite a complex 129Xe demagnetisation and replenishment regime which will be further optimised.
This is ongoing work, and future activities will also focus resolving other spectral peaks in the brain and utilizing the high spectral resolution to map brain oxygenation. Also, this method enables voxel-wise quantification of HP 129Xe dissolved in various biochemical compartments in the brain and is the scope of future studies.
Conclusion
In this work, we have demonstrated isotropic 3D spectroscopic imaging of HP 129Xe dissolved in the brain in with an acquired voxel size of (2cm)3 and interpolated to a voxel size of (0.625 cm)3.Acknowledgements
Funding from MRC grant MR/M008894/1.References
1. Rao MR, Stewart NJ, Griffiths PD, Norquay G, Wild JM. Imaging Human Brain Perfusion with Inhaled Hyperpolarized (129)Xe MR Imaging. Radiology 2018;286(2):659-665.
2. Rao MR, Norquay G, Stewart NJ, Hoggard N, Griffiths PD, Wild JM. Assessment of brain perfusion using hyperpolarized (129) Xe MRI in a subject with established stroke. J Magn Reson Imaging 2019;0(0).
3. Hane FT, Li T, Plata J-A, Hassan A, Granberg K, Albert MS. Inhaled Xenon Washout as a Biomarker of Alzheimer’s Disease. Diagnostics 2018;8(2):41.
4. Shepelytskyi Y, Hane FT, Grynko V, Li T, Hassan A, Albert MS. Hyperpolarized 129Xe Time-of-Flight MR Imaging of Perfusion and Brain Function. Diagnostics 2020;10(9):630.
5. Rao M, Stewart NJ, Norquay G, Griffiths PD, Wild JM. High resolution spectroscopy and chemical shift imaging of hyperpolarized (129) Xe dissolved in the human brain in vivo at 1.5 tesla. Magn Reson Med 2016;75(6):2227-2234.
6. Rao MR, Norquay, G., Steward, N.J., Wild, J.W.,. Measuring 129Xe transfer across the blood brain barrier using magnetic resonance spectroscopy. Magnetic Resonance in Medicine.
7. Kilian W, Seifert F, Rinneberg H. Dynamic NMR Spectroscopy of Hyperpolarized 129Xe in Human Brain Analyzed by an Uptake Model. Magnetic Resonance in Medicine 2004;51(4):843-847.
8. Greiser A, von Kienlin M. Efficient k-space sampling by density-weighted phase-encoding. Magnetic Resonance in Medicine 2003;50(6):1266-1275.
9. Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM. 129Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Physical Review Letters 2018;121(15):153201.
Figures