3566
Reproducibility Study Measuring Ventilation, Gas Exchange and Surface-to-Volume Using Hyperpolarized Xenon in Free-breathing Human Subjects1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
We have previously shown the advantages of multi- over single-breath imaging of lung ventilation using HP gas MRI, and have recently introduced our approach for comprehensively assessing the lung function during a tidal breathing scheme to quantify ventilation and dissolved parameters of xenon distribution. Here, we presented the repeatability of the imaging markers in a healthy subject and a patient with severe chronic obstructive pulmonary disease (COPD) with persisting cough. We have shown that the technical back-to-back reproducibility of measuring lung function with our scheme is excellent in a healthy subject and satisfactory in a COPD subject with persisting cough.
Introduction
We previously demonstrated the advantages of multi- over single-breath imaging of lung ventilation using hyperpolarized (HP) gas MRI1, showing that large regions of the lung which appear entirely unventilated after one breath in patients with severe disease are instead ventilated very slowly2. To more directly visualize gas, blood and their exchange, we developed an imaging method based on repeated inhalation of hyperpolarized xenon (HXe) during tidal breathing, in which we measure both ventilation and dissolved parameters of xenon distribution. Despite satisfactory precision in trained healthy subjects, however, some variability in tidal breathing remained unavoidable. Recently3, we reduced the technical complexity of our multibreath MRI protocol by analytically correcting for the breath-by-breath variability using flow measurements with pneumotachs. Subjects now inhale/exhale freely while a small amount of HP gas is injected into the breathing line; we also increased the number of breaths to >60 for each image set. Here, we demonstrate the repeatability of our imaging markers in a healthy subject and a patient with severe chronic obstructive pulmonary disease (COPD) with persisting cough.Methods
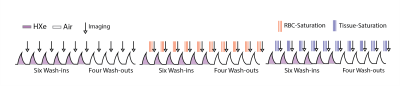
Figure 1 shows a protocol schematic: subjects breathe freely through a mouthpiece while an additional, constant amount (50-100 mL) of HXe (wash-in) or air (wash-out) is injected into the line right before inhalation. The scheme includes 6 ad libitum normoxic wash-in breaths of hyperpolarized gas (129Xe:O2:N2 ~1:2:7) followed by 4 washout breaths of room air. A GRAPPA-accelerated image was performed at each exhale, triggered by subjects’ breathing pattern. 87%-enriched Xe gas was polarized using a commercial device (XeBox-E10; Xemed, LLC) providing polarizations of ~40%; subjects were imaged in a whole body 1.5-T MRI system (Siemens MAGNETOM) using a flexible 8-channel chest coil (Stark Contrast). We employed a 2D multi-slice GRE image set with 6×~25mm coronal slices adjusted to cover the whole lung with a 20% inter-slice gap, MS=6x64x48, FOV=40x30cm2, a≈6°, TR/TE=7.0/3.3ms. The 10-breath wash-in/washout series was then repeated five more times with additional series encoding gas dissolution and uptake. The problem of imaging very small dissolved xenon signals can be alleviated using Xenon polarization Transfer Contrast (XTC), which applies saturation pulses to destroy sselectively the signal fractions, and in the chosen compartment at each end-exhale. The series were further analyzed using a model of voxel-wise per-breath gas replacement:V0,EE=FRC, Vi,EI = Vi-1,EE+Ii(TV/TV), Vi,EI-Ei(TV/TV),
S0=0, Si=(Si-1+TV×S∞)×(Vi,EE/Vi,EI)×fRF×fO2×fRBC×ftissue (eq.1)
On the first line, FRC and TV are fit parameters describing the average regional gas volume and average change during breathing, respectively; Vi,EE/EI track that region’s volumes as volumes, Ii and Ei are inhaled and exhaled, and TV refers to the average inhaled/exhaled volume as measured using pneumotach flowmeters. The second line describes the evolution of signal as a volume, TV, containing additional signal TV x S∞ is inhaled, the fraction Vi,EE/Vi,EI of signal is lost via exhalation, and independently measured factors, fRF and fO2 diminish signal between breaths through RF-induced and collisional O2-induced relaxation mechanisms. fRBC and ftissue account for the fraction of signal lost by applying RF to the hemoglobin-bound and tissue/plasma-dissolved resonances, respectively. The fit to the complete time series is then used to determine local FRC and TV, the voxel’s gas volumes, and factors dependent on gas exchange. We then define the gas exchange rate in terms of the rate at which these inverted spins re-enter the gas phase during the time, TR, between saturation pulse:
χ = (3/4) × (1 - fRBC)1/N × (FRC / TR) (eq.2)
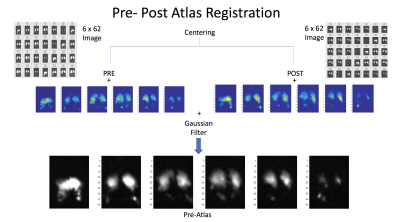
In this expression, (1 - fRBC)1/N represents gas signal loss for each of the N saturation pulses. To make this reframing of fRBC useful, we choose a TR between saturation pulses that both imposes sensitivity to physiologically relevant gas exchange and makes rapid enough contrast feasible during tidal breathing (8ms, TR=30ms saturation pulses). ftissue can be similarly interpreted as local surface-to-volume ratio4, but here we use it to correct for a small off-resonance excitation of Xe in tissue/plasma when measuring . All HXe images were coregistered with an affine transformation and then added to produce a lung atlas. Finally, all pre-post images were registered to the common reference lung atlas (Figure 2).
Results and Discussion
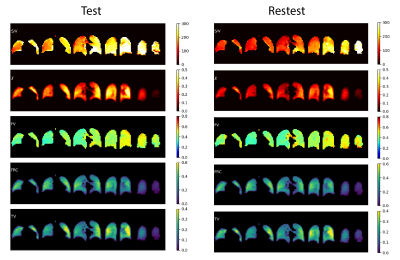
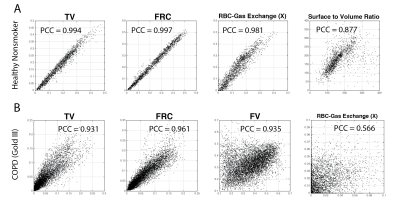
Independent analysis of the two multibreath image sets yield the functional maps shown in Figure 3, showing excellent repeatability with near-identical features in a healthy subject. Figure 4A shows test-retest voxel-by-voxel correlation for all functional maps estimated in the healthy subject. The correlation coefficient was in the range of 0.88–0.99 for all estimated functional maps, demonstrating high technical repeatability of the quantified markers for ventilation and dissolved parameters of xenon distribution. The Stage III COPD subject successfully performed the breathing scheme, but unintentionally coughed at times during the 60 breaths. Despite this, results in Figure 4B for the COPD subject show fair repeatability in measures of ventilation and a moderate correlation in measures of gas exchange, with more variability in both technical reproducibility and lung physiology of gas exchange.Conclusion
The technical back-to-back reproducibility of measured lung function in our multibreath tidal breathing scheme is excellent in a healthy subject and satisfactory in a COPD subject with persisting cough. Future studies will decouple this technical repeatability from real physiological variability.Acknowledgements
No acknowledgement found.References
[1] Hamedani H, Clapp JT, Kadlecek SJ, Emami K, Ishii M, Gefter WB, et al. Regional Fractional Ventilation by Using Multibreath Wash-in 3He MR Imaging. Radiology. 2016;279(3):917–24.
[2] H Hamedani, F Bermudez, R Baron, S Kadlecek, K Ruppert, Y Xin, S Siddiqui, M Pourfathi, F Amzajerdian, L Loza, T Achekzai, I Duncan, M Cereda, F Sertic, RR Rizi. A Comparison of Hyperpolarized Gas Imaging of Aeration and Fractional Ventilation. American Thoracic Society C108. COPD: PHENOTYPE, MECHANISM, AND TREATMENT. A5772-A5772
[3] H Hamedani, S Kdlecek, R Baron, K Ruppert, Y Xin, F Amzajerdian, L Loza, M Pourfathi, I Duncan, TS Achekzai, S Siddiqui, I Duncan, M Cereda, RR Rizi. Measuring Ventilation with Hyperpolarized Gas During Normal Breathing. American Thoracic Society. D110. NEW INSIGHTS FROM ADVANCED IMAGING. A7904-A7904
[4] Muradyan I, Butler JP, Dabaghyan M, Hrovat M, Dregely I, Ruset I, et al. Single breath xenon polarization transfer contrast (SB-XTC): implementation and initial results in healthy humans. J Magn Reson Imaging JMRI 2013;37:457–70. https://doi.org/10.1002/jmri.23823.
Figures