3565
Feasibility of Xenon Polarization Transfer Contrast Imaging using Continuous RF Irradiation1University of Pennsylvania, Philadelphia, PA, United States
Synopsis
Xenon-polarization Transfer Contrast (XTC) imaging is a powerful technique for quantifying exchange rates between gas- and dissolved-phase xenon compartments and involves a series of radiofrequency (RF) saturation pulses applied to the targeted dissolved-phase resonance. Although increasing the number of pulses and their spacing generates greater contrast, it also increases acquisition time. In this work, in an effort to reduce acquisition time, particularly for free-breathing imaging protocols, we explored the use of continuous RF irradiation to depolarize dissolved-phase xenon, with the goal of producing results similar to those achieved with pulsed saturations, but in a significantly shorter time period.
Introduction
Hyperpolarized xenon-129 (HXe) MRI provides a valuable tool for assessing disease-associated changes in lung structure and function by measuring the distinct chemical shifts HXe experiences when dissolved in the tissue/plasma (TP) or bound to red blood cells (RBCs). Direct imaging of these dissolved-phase (DP) components is often challenging, since only approximately 2% of HXe is dissolved at any given time and certain lung diseases, such as emphysema, decrease gas exchange, further reducing available signal. An alternative approach, Xenon-polarization Transfer Contrast (XTC) imaging, indirectly quantifies DP xenon by taking advantage of the high gas-phase (GP) signal1. By saturating the targeted DP resonance, the GP is depolarized proportionally to the exchange between those components. Traditionally, multiple saturation pulses interspersed with specific delay times to allow for gas exchange were used to achieve sufficient contrast. This approach is time inefficient, however, and prolongs the acquisitions due to the need for an extended contrast preparation period. In this work, we explored the feasibility of using long, compartment-selective RF pulses2,3,4 for faster, more efficient saturation in healthy and emphysematous rats.Methods
Imaging was performed in 4 male Wistar rats (300 – 400 g) according to protocols approved by the Institutional Animal Care and Use Committee. In one animal, emphysema was induced via a localized elastase injection (100 µL/g) approximately one month prior to imaging. Animals were ventilated with room air and then switched to an 80% HXe, 20% oxygen mixture for 4 wash-in breaths, followed by imaging during a 10 second breath-hold. All imaging adhered to SAR limitations and was performed on a 1.5 T scanner (Magnetom Avanto, Siemens) with a custom xenon-129 birdcage coil (Stark Contrast, Germany). A prototype commercial system (XeBox-E10, Xemed LLC, NH) was used to polarize 87% enriched xenon-129.XTC data sets were obtained from three consecutive 2D GP GRE projection images (S1, S2, and S3). Imaging parameters included: flip angles (αi) of 4°, 9°, and 13°, respectively, TR/TE = 8.1/3.9 ms, matrix size = 36x48, and FOV of 60 mm. For continuous RF acquisitions, a series of rectangular saturation pulses with varying lengths (25-100 ms) and flip angles (1,800°-54,000°) was applied on-resonance (213 ppm for RBC or 199 ppm for TP) in-between the second pair of images to generate contrast, and off-resonance (-213 ppm for RBC or -199 ppm for TP) between the first pair as a control for apparent T1 decay and any inadvertent GP magnetization loss. Due to hardware limitations, 500 ms and 1 s saturation pulses had to be implemented by repeating 100 ms pulses with minimal separation (< 0.3 ms). For pulsed saturations, 60 8 ms Gaussian 180° RF pulses with 30 ms spacing were used. Depolarization ratio maps, RDepol, were calculated from the ratio of these images, as shown in the equation below, where RCorr and RExch are the T1-correction and exchange-weighted depolarization maps, respectively, adjusted for excitation flip angle and number of phase-encoding lines (PE).
$$ R_{Depol}=\frac{R_{Exch}}{R_{Corr}} =\frac{S_3 S_1}{S_2^2} (\frac{sin^2 \alpha_2}{sin \alpha_1sin \alpha_3})(\frac{cos \alpha_1}{cos \alpha_3})^{PE/2} $$
Results and Discussion
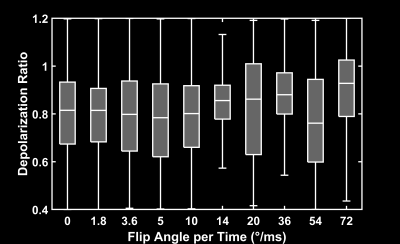
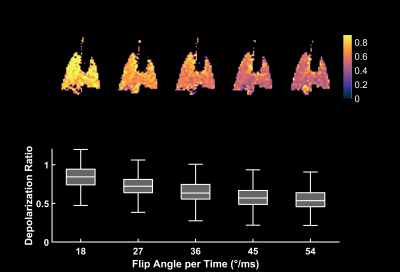
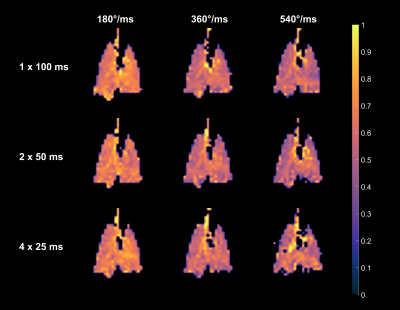
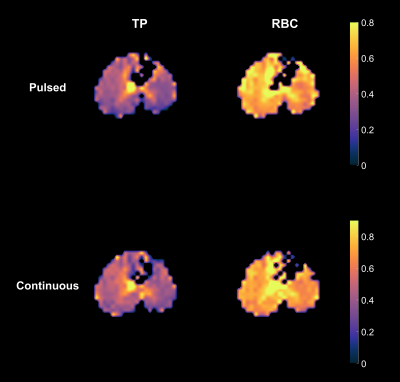
Figure 1 depicts the depolarization ratios associated with the control saturation scheme, RCorr, illustrating the minimal impact of the off-resonance continuous saturations on the GP magnetization in a healthy rat. An average RCorr of 0.82 for 500 ms saturation (five 100 ms pulses) with flip angles ranging from 1.8°/ms to 72°/ms—compared to 0.80 seen with a 0° flip angle—suggests that these continuous pulses do not directly depolarize the GP. Figure 2 illustrates the effect of increasing flip angle on the depolarization of the TP resonance in another healthy rat. There is a clear trend despite the long saturation time, which allows xenon to exchange repeatedly between the various compartments and introduces uncertainty with regards to the flip angle that each xenon atom experiences. In order to demonstrate consistency and RF amplifier stability, very large flip angles were evaluated. As seen in Figure 3, saturations with the same flip angle per pulse length at various flip angle and pulse length combinations produced similar depolarization levels. In Figure 4, discrete and continuous saturation schemes are compared in an emphysema rat model. Due to the lower than expected xenon gas exchange in this model, 1 s of saturation (ten 100 ms pulses) was applied at a rate of 18°/ms. The distributions were consistent between the two methods with average depolarization ratios of 0.42 ± 0.18 and 0.49 ± 0.18 for TP and 0.70 ± 0.16 and 0.75 ± 0.16 for RBC, respectively. However, the continuous saturation scheme significantly reduced overall acquisition time by 45%, displaying significant promise for use in free-breathing imaging protocols, especially with further optimizations of imaging and saturation parameters.Conclusion
XTC MRI with continuous RF irradiation produces depolarization distributions similar to those obtained with pulsed saturation schemes, but with a significant time advantage that decreases required breath-hold durations.Acknowledgements
No acknowledgement found.References
[1] Ruppert, Kai, et al (2000). Probing lung physiology with xenon polarization transfer contrast (XTC). MRM 44(3), 349-357.
[2] Amzajerdian, Faraz, et al (2020). Measuring pulmonary gas exchange using compartment‐selective xenon‐polarization transfer contrast (XTC) MRI. MRM 00, 1-14.
[3] Sun, Philip, et al (2011). Simulation and optimization of pulsed radio frequency irradiation scheme for chemical exchange saturation transfer (CEST) MRI—demonstration of pH-weighted pulsed-amide proton CEST MRI in an animal model of acute cerebral ischemia. MRM 66(4), 1042-1048.
[4] Kunth, Martin, et al (2015). Continuous-wave saturation considerations for efficient xenon depolarization. NMR in Biomedicine 28(6) 601-606.
Figures