3564
Temporal correlation of alveolar-capillary 129Xe signal dynamics with the cardiac cycle1Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield, United Kingdom
Synopsis
Global 129Xe MRS was acquired in the lungs synchronously with an ECG recording to enable association of dissolved-phase 129Xe signal dynamics with the cardiac cycle. Time-domain Voigt fitting was used to calculate resonance parameters corresponding to 129Xe in red blood cells and tissue/blood plasma in the lungs. Positive and negative signal changes of 129Xe in the blood were found to be associated with ventricular systole and diastole in a healthy volunteer.
Introduction
MR imaging and spectroscopy of hyperpolarised 129Xe dissolved in pulmonary red blood cells (RBCs) and parenchymal tissue/plasma (TP) is of interest for investigating a range of cardiopulmonary diseases (1). It has been observed previously that while the 129Xe-TP signal is relatively stable during MR protocols, the 129Xe-RBC signal oscillates at a frequency consistent with cardiac pulsation (2-5). Furthermore, it has been shown that the observed RBC oscillations can be used to distinguish 129Xe gas exchange dynamics between patients with obstructive pulmonary disease (COPD), idiopathic pulmonary fibrosis (IPF), left heart failure (LHF) and pulmonary arterial hypertension (PAH) (6). In this study, the aim is to evaluate these observed RBC oscillations and correlate them with specific time points in the cardiac cycle by mapping 129Xe-RBC signal dynamics to an ECG waveform during an MR protocol.Methods
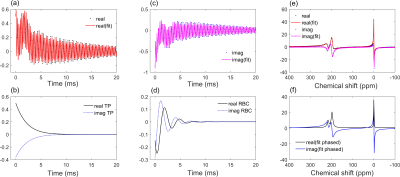
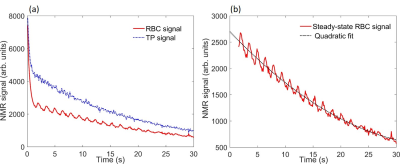
Hyperpolarised 129Xe pulmonary MRS was performed on a healthy male volunteer on a clinical 1.5 T (GE, HDx) scanner with a quadrature flex T-R coil (CMRS). The scan was performed at breath-hold apnoea after inhalation (from functional residual capacity) of 600 mL of hyperpolarised 129Xe (7) balanced with 400 mL of medical-grade N2. An ECG signal was recorded throughout the scan duration (Fig. 4). The sequence had the following parameters: soft pulse designed to minimise 129Xe-gas excitation centred on the 129Xe-TP resonance with a flip angle = 30°, sample points = 512, receive bandwidth = 15 kHz, TR = 100ms. The flip angle was chosen such that the dissolved-phase 129Xe signal was being sampled at steady-state after ~750 ms of transit through the alveolar-capillaries. The flip angle was determined by applying the flip-angle-TR equivalence formula (8,9) FA = cos-1[(1-TRf)], where here f=[1-cos(α)]/τ=1/τ is defined for a flip angle of 90° and τ = 750 ms is the time the dissolved-phase 129Xe magnetisation is available before being destroyed. Data analysis was performed by fitting the real and imaginary parts of the time-domain signal to a 3-resonance Voigt function (Fig. 1) (5) defined by (10) $$\text{Re}[\nu(t)]=\sum_{n=1}^3A_{n}\exp(-\pi\Gamma_{n}^lt)\exp\left[-\left(\frac{\pi\Gamma_{n}^gt}{2\sqrt{\ln2}}\right)^2\right]\cos[(\omega_{0,n}-\omega_{t})t+\phi_{n}]\quad\quad\quad \\ \text{Im}[\nu(t)]=\sum_{n=1}^3A_{n}\exp(-\pi\Gamma_{n}^lt)\exp\left[-\left(\frac{\pi\Gamma_{n}^gt}{2\sqrt{\ln2}}\right)^2\right]\sin[(\omega_{0,n}-\omega_{t})t+\phi_{n}]\quad\quad(1)$$ where A is the FID the amplitude, Γl and Γg are Lorentzian and Gaussian linewidths, ω0-ωt =Δω is the frequency difference between the 129Xe resonance frequencies and the RF transmit/receive frequency and φ is the phase of the (Gas,TP,RBC) 129Xe resonant peaks.The steady-state signal amplitude from the RBC signal was normalised for gas reservoir magnetisation decay by dividing by a quadratic function fitted to the decaying RBC signal (Fig. 2 (b)).
Results and Discussion
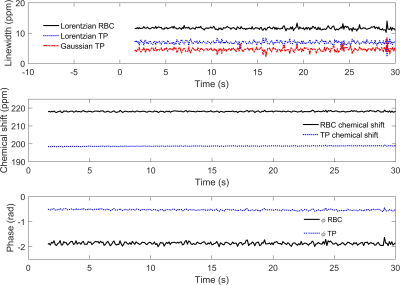
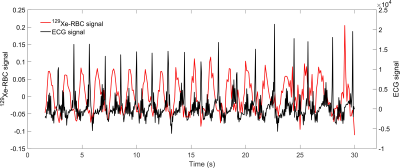
The RBC signal amplitude was measured to oscillate with a mean fractional peak-to-peak signal change of 0.142 ± 0.008 at a frequency of 0.63 Hz, consistent with the subject’s heart rate of ~40 beats/min (Fig. 4). As shown in Fig. 3, none of the other fitted FID parameters exhibited detectable periodic oscillations during the breath-hold within their respective experimental errors (Fig. 3). Mean fitted parameter values: RBC:TP = 0.63 ± 0.04; RBC Γl = 11.6 ± 0.4 ppm; RBC and TP chemical shifts of 218.1 ± 0.3 ppm 198.7 ± 0.2 ppm (gas resonance at 0 ppm for reference). The Lorentzian and Gaussian linewidths calculated for the TP resonance were Γl = 6.9 ± 0.5 ppm and Γg = 4.6 ± 0.6 ppm, giving a Voigt parameter of Γl /Γg = 1.5, consistent with previous findings (5) that the TP resonance has a significant Gaussian component to its lineshape.In Fig. 4, the fractional RBC amplitude oscillation is overlaid against the recorded ECG signal, where it can be seen that the RBC maxima and minima occur respectively during ventricular systole and diastole, confirming that during systole, the faster blood flow and the increased capillary volume/surface area contributes to the higher observed 129Xe RBC signal. While this work was performed on inhalation at functional residual capacity (FRC), previous work has shown that the RBC oscillations are more pronounced at total lung capacity (TLC) (3); since the alveolar air pressure in the lung at TLC is higher than at FRC, it is likely that more capillaries are restricted or that the capillary diameter is smaller during diastole (11), meaning the relative changes in RBC should be dependent on lung inflation level. In patients with pathological changes in vessel compliance, for example in patients with PAH, where there is a potential phase difference between the heart beat and the delivery of pressure waves to the pulmonary capillaries, combining ECG measurements with 129Xe spectroscopy could be used to detect these differences globally as well as regionally (12).
Conclusions
In this work, ECG was synchronised with global 129Xe MRS to temporally correlate 129Xe RBC signal dynamics with the cardiac cycle. We found that positive and negative changes in the RBC signal amplitude occur during ventricular systole and diastole in a healthy volunteer.Acknowledgements
This work was funded by the University of Sheffield, Medical Research Council (MR/ M008894/1) and the National Institute for Health Research (NIHR-RP-R3-12-027). The views expressed in this publication are those of the authors and not necessarily those of the National Health Service, the National Institute for Health Research or the Department of Health.References
1. Eddy RL, Parraga G. Pulmonary xenon-129 MRI: new opportunities to unravel enigmas in respiratory medicine. European Respiratory Journal 2020;55(2):1901987.
2. Venkatesh AK, Hong KS, Kubatina Y, Sun RV, Mulkern FA, Jolesz MS, Albert MS. Direct Observation of the Transport of 129Xe from the Lung-Gas to the Tissue and the Blood. Proc Intl Soc Mag Reson 2001:984.
3. Ruppert K, Altes TA, Mata JF, Ruset IC, Hersman FW, Mugler JP. Detecting pulmonary capillary blood pulsations using hyperpolarized xenon-129 chemical shift saturation recovery (CSSR) MR spectroscopy. Magnetic Resonance in Medicine 2016;75(4):1771-1780.
4. Norquay G, Leung G, Stewart NJ, Wolber J, Wild JM. 129Xe chemical shift in human blood and pulmonary blood oxygenation measurement in humans using hyperpolarized 129Xe NMR. Magnetic Resonance in Medicine 2017;77(4):1399-1408.
5. Bier EA, Robertson SH, Schrank GM, Rackley C, Mammarappallil JG, Rajagopal S, McAdams HP, Driehuys B. A protocol for quantifying cardiogenic oscillations in dynamic (129) Xe gas exchange spectroscopy: The effects of idiopathic pulmonary fibrosis. NMR in biomedicine 2019;32(1):e4029-e4029.
6. Wang Z, Bier EA, Swaminathan A, Parikh K, Nouls J, He M, Mammarappallil JG, Luo S, Driehuys B, Rajagopal S. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. European Respiratory Journal 2019;54(6):1900831.
7. Norquay G, Collier GJ, Rao M, Stewart NJ, Wild JM. 129Xe-Rb Spin-Exchange Optical Pumping with High Photon Efficiency. Physical Review Letters 2018;121(15):153201.
8. Gómez Damián PA, Sperl JI, Janich MA, Khegai O, Wiesinger F, Glaser SJ, Haase A, Schwaiger M, Schulte RF, Menzel MI. Multisite Kinetic Modeling of (13)C Metabolic MR Using [1-(13)C]Pyruvate. Radiol Res Pract 2014;2014:871619-871619.
9. Ruppert K, Amzajerdian F, Hamedani H, Xin Y, Loza L, Achekzai T, Duncan IF, Profka H, Siddiqui S, Pourfathi M, Sertic F, Cereda MF, Kadlecek S, Rizi RR. Assessment of flip angle–TR equivalence for standardized dissolved-phase imaging of the lung with hyperpolarized 129Xe MRI. Magnetic Resonance in Medicine 2019;81(3):1784-1794.
10. Bruce SD, Higinbotham J, Marshall I, Beswick PH. An Analytical Derivation of a Popular Approximation of the Voigt Function for Quantification of NMR Spectra. Journal of magnetic resonance 2000;142(1):57-63.
11. Hughes JM, Glazier JB, Maloney JE, West JB. Effect of lung volume on the distribution of pulmonary blood flow in man. Respiration physiology 1968;4(1):58-72. 1
12. Niedbalski PJ, Bier EA, Wang Z, Willmering MM, Driehuys B, Cleveland ZI. Mapping cardiopulmonary dynamics within the microvasculature of the lungs using dissolved 129Xe MRI. Journal of applied physiology 2020;129(2):218-229.
Figures