3533
Retrospective Eddy Current Artifact Reduction for Balanced SSFP Cine Imaging via Deep Learning
Cynthia Chen1, Christopher Sandino2, Adam Bush3, Frank Ong2, and Shreyas Vasanawala3
1California Institute of Technology, Pasadena, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States
1California Institute of Technology, Pasadena, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States
Synopsis
Eddy currents due to changing magnetic fields reduce diagnostic image quality, especially in SSFP acquisitions. In this work, we propose a deep learning method that successfully reduces eddy current artifacts in 2D cardiac cine imaging using a 3D U-Net architecture. Our method is completely retrospective and does not require any sequence or hardware modifications.
Introduction
Balanced steady-state free precession (SSFP) achieves high signal-to-noise ratio and contrast-to-noise ratio while allowing for fast image acquisition. However, SSFP sequences are sensitive to eddy currents caused by changing phase-encoding gradients, which is particularly problematic for golden ratio sampling schemes1 and accelerated acquisitions that undersample k-space using pseudo-random phase encoding orderings.2 Eddy currents can be mitigated by designing orderings with smoother transitions (i.e. pairing),2 however, these require sacrifices to sampling efficiency. Another technique uses RF phase cycling to cancel out perturbations predicted by impulse response-based modeling,3 although this requires pulse sequence modifications and a calibration scan to measure the impulse response for each scanner. To this end, we propose a deep learning approach based on a 3D U-Net4 architecture to retrospectively reduce eddy current artifacts in highly undersampled 2D cardiac cine data.Methods
Data Collection & Simulation: With IRB approval, 21 fully-sampled 2D cardiac cine datasets free of eddy current artifacts were collected to serve as ground truth. These datasets are retrospectively undersampled by a factor of 12 using a variable-density k-t sampling pattern.5 Since eddy currents manifest as random signal fluctuations in SSFP, artifacts are simulated by scaling these k-space lines by random amplitudes in random ranges varying from very corrupted (0,2) to ground truth (1,1). Figure 1 shows simulated examples with increasing eddy current artifacts. Both corrupted and non-corrupted undersampled k-space datasets were then reconstructed using a model-based deep learning network6 to form the input-output pairs for network training.Network Architecture: To keep consistent dimensions for training, images were padded to 224x224x32 (height, width, time frames). Data augmentation was performed by flipping the images vertically and horizontally. We used a 3D U-Net architecture with an additive skip connection to perform residual learning. Figure 2 shows the data processing pipeline, eddy current simulation, and U-Net architecture. The model was trained using an Adam optimizer,7 Mean Squared Error (MSE) loss function, and a batch size of 32 for 50 epochs, which took approximately 3 hours.
Evaluation: The eddy current artifact reduction network is evaluated by computing structural similarity index (SSIM) between network output and ground truth pairs in the training and validation sets. The network is tested on a prospectively collected 2D cardiac cine dataset with 12X acceleration and severe eddy current artifact.
Results
We observed successful reduction of eddy current artifacts in both simulated data and prospectively acquired undersampled cine data, as shown in Figures 3 and 4. Quantitatively, the predicted images for simulated data achieved a higher average SSIM than the corrupted images when using the ground truth as reference (see Figure 5). For prospectively acquired data, the blinking eddy current artifacts present over time in the original data were also significantly reduced in the predictions.Discussion & Conclusion
This work demonstrates the effectiveness of using a neural network to retrospectively reduce eddy current artifacts in cine MRI. Eddy currents are currently a limiting factor for sequence choices because eddy current artifacts lead to reduced diagnostic image quality, but retrospectively reducing these artifacts allows for sequence designs with more optimal view ordering and with higher acceleration factors to be used.Two main benefits of this method are that sequence modifications and large amounts of data for training are not needed because training data is generated through simulations. Additionally, this method is not limited to eddy current artifact reduction for cine imaging and can also be extended to reduce artifacts in other imaging techniques that involve high gradient slewing.
Acknowledgements
This work was supported by the Caltech SURF Program, NIH; Contract grant numbers: R01EB009690, NIH R01EB026136, and GE Healthcare.References
- Sayin O, Derbyshire JA, McVeigh E, Herzka D. Through-slice dephasing for eddy current artifact reduction in bSSFP. J Cardiovasc Magn Reson. 2012;14(Suppl 1):P271. Published 2012 Feb 1. doi:10.1186/1532-429X-14-S1-P271
- Bieri O, Markl M, Scheffler K. Analysis and compensation of eddy currents in balanced SSFP. Magn Reson Med. 2005;54(1):129-137. doi:10.1002/mrm.20527
- Bruijnen T, Stemkens B, van den Berg CAT, Tijssen RHN. Prospective GIRF-based RF phase cycling to reduce eddy current-induced steady-state disruption in bSSFP imaging. Magn Reson Med. 2020;84(1):115-127. doi:10.1002/mrm.28097
- Han Y, Sunwoo L, Ye JC. k -Space Deep Learning for Accelerated MRI. IEEE Trans Med Imaging. 2020;39(2):377-386. doi:10.1109/TMI.2019.2927101
- Lai P, Brau A. Improving cardiac cine MRI on 3T using 2D k-t accelerated auto-calibrating parallel imaging. J Cardiovasc Magn Reson. 2014;16(Suppl 1):W3. Published 2014 Jan 16. doi:10.1186/1532-429X-16-S1-W3
- Sandino CM, Lai P, Vasanawala SS, Cheng JY. Accelerating cardiac cine MRI using a deep learning‐based ESPIRiT reconstruction. Magn Reson Med. 2021;85(1):152-167. doi:10.1002/mrm.28420
- Kingma DP, Ba J. Adam: A Method for Stochastic Optimization. 2014. arXiv:1412.6980
Figures
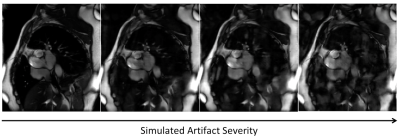
Figure 1. Simulated eddy current artifacts increasing in severity from left to right.
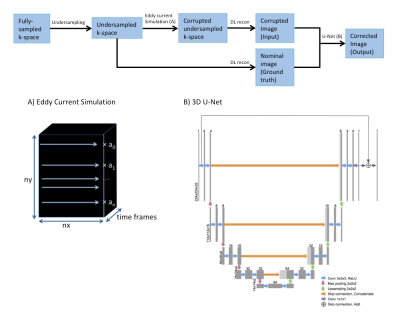
Figure 2. Data processing pipeline. A) Eddy current effects of random phase encoding orderings were simulated by scaling each k-space line by a random amplitude in a randomly chosen range. B) 3D-Unet Architecture with an additive skip connection to enable residual learning. The input and output sizes are Nx224x224x32.
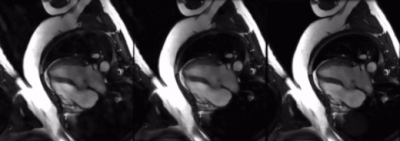
Figure 3. Comparison of simulated corrupted images (left), predicted images by the neural network (middle), and nominal ground truth images (right). Eddy current artifacts are greatly reduced in the prediction.
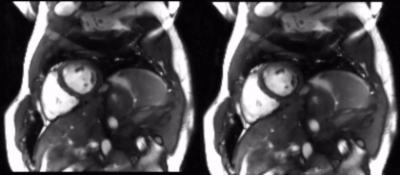
Figure 4. Prospectively acquired data with optimal view ordering but apparent eddy current artifacts and prediction following application of 3D U-Net. (Left) A 2D cardiac cine dataset is prospectively acquired with 12X acceleration, then reconstructed using a model-based deep learning approach. Eddy current artifacts manifest as residual aliasing across the image. (Right) The images on the right are corrected using our 3D U-Net approach, resulting in reduction of eddy current artifacts.
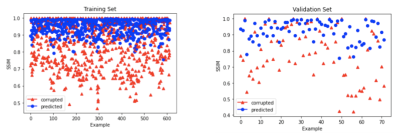
Figure 5. SSIM for corrupted data (red) and predictions (blue). Neural network predictions achieve a higher average SSIM than corrupted data for both training and validation. (Left) Training set examples with an average SSIM of 0.9476 for predicted images and 0.8313 for corrupted images. (Right) Validation set examples with an average SSIM of 0.9231 for predicted images and 0.7811 for corrupted images.