3522
Aliasing Artifact Reduction in Spiral Real-Time MRI1Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Department of Linguistics, University of Southern California, Los Angeles, CA, United States
Synopsis
Mid-sagittal spiral MRI of speech production often suffers from a distinct and disruptive aliasing artifact arising from a spurious signal outside the FOV. In this work, we determine that the spurious signal is caused by gradient nonlinearity and an ineffective anti-aliasing filter in spiral readout. We propose and evaluate two methods to mitigate the artifact, termed the large FOV (LF) method and the estimation-subtraction (ES) method. Qualitative evaluation score from two speech experts using a 5-level Likert scale improved 1.25 and 1.35 with 228.8% and 6.9% increment of reconstruction time for the LF and ES methods, respectively.
Introduction
Real-time MRI is an established tool to study the dynamics of speech articulators during human speech production. K-space data is usually collected with a spiral trajectory to leverage its high sampling efficiency. However, this data acquisition suffers from a distinct artifact in the mid-sagittal slice. Gradient nonlinearity can cause large image distortion and signal enhancement at locations far away from the magnet iso-center (1,2). Furthermore, the readout anti-aliasing filter does not provide FOV restriction in trajectories (including spiral) where the direction is changing within one readout (3,4). These two effects combine and cause a signal hotspot outside the desired FOV, which leads to aliasing artifact within the FOV. The artifact can overlap with important articulators, degrade the image quality and negatively affect the visualization of speech production.In this study, we demonstrate the cause of the spiral aliasing artifact using simulations. We then propose two methods to mitigate the artifact, termed the “large FOV” (LF) method and the “estimation-subtraction” (ES) method. The LF method reconstructs a large FOV including the aliasing source during reconstruction, and the ES method estimates k-space data from the artifact source and then subtracts it from the acquired multi-channel raw data. We evaluate both methods in the content of real-time speech production MRI. Fifty-eight subjects were involved, and two speech experts were asked to give qualitative scores for artifact severity using a 5-level Likert scale.
Methods
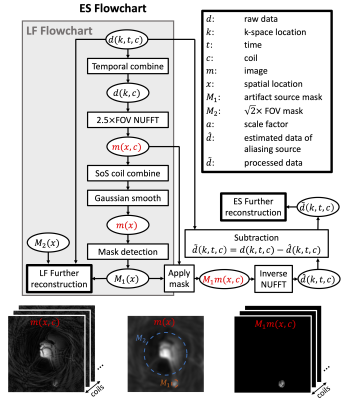
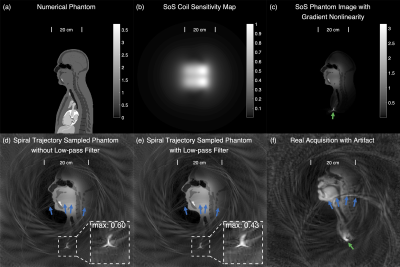
Simulation. A mid-sagittal slice extracted from the XCAT phantom (5) was used to demonstrate the origin of the artifact. Sensitivity maps were simulated using Biot-Savart law. A gradient profile of the Zoom coil (GE Healthcare, Waukesha, Wisconsin) in the form of spherical harmonic was used to simulate for gradient nonlinearity. We simulated a dwell time of 1 μs, and then sub-sampled the k-space to a dwell time of 4 μs to allow for simulation of the readout low-pass filter effects.Artifact Reduction Methods. The artifact reduction methods are illustrated in Figure 1. The first step in both methods is to identify the aliasing source M1, which is done on temporally combined, 2.5×FOV reconstructed image. LF Method: a 2.5×FOV is kept during reconstruction and a circular $$$\sqrt{2}$$$×FOV mask M2 and M1 are applied to reconstruct the signal within the masks. Since the designed trajectory does not support 2.5×FOV, applying M2 can reduce blurring caused by enlarged reconstruction FOV. Application of M1 will ensure a faithful reconstruction of the aliasing source, reducing its aliasing into undesired locations. ES method: the multi-coil k-space data of M1 is estimated by adjoint NUFFT. This k-space signal is subtracted from the acquired raw data to allow for aliasing reduced reconstruction.
Data Acquisition. Fifty-eight subjects were scanned using the MRI protocol presented in (6), and the stimuli of phonologically rich sentences was used in this study. A real-time speech MRI system (7) was used to collect the data, including a 1.5T Signa Excite scanner (GE Healthcare, Waukesha, Wisconsin), a custom 8-channel upper airway receive coil, and an imaging platform RT-Hawk (Heart Vista, Los Altos, California) (8). A 13-interleaf spiral-out trajectory supporting a 20×20cm2 FOV with a bit-reversed temporal sampling order (9) was used. Scan parameters include: TR/TE=6.0/0.8ms, voxel size=2.4×2.4×6mm3, bandwidth=±125kHz, flip angle=15o. Images were reconstructed to 12ms/frame by using a temporally constrained reconstruction (10).
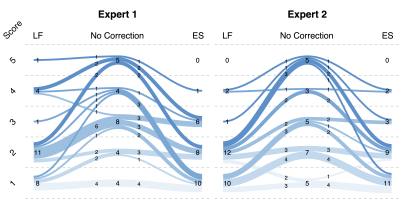
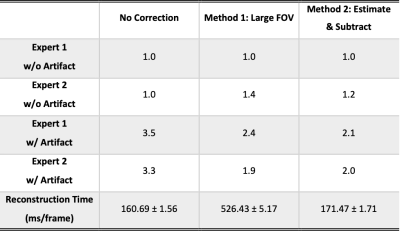
Evaluation. The fifty-eight scans were reconstrued using three methods: no correction, LF correction, and ES correction. In the initial evaluation, a threshold:$$$\rm{SI_{out}^{max}/SI_{in}^{max}}\leq0.4$$$(highest signal intensity outside/within FOV) was determined to identify datasets without apparent artifact and 4 datasets were excluded from the following evaluations. Then, two speech experts were asked to perform a two-round evaluation of the artifact severity by grading the provided videos with a 5-level Likert score, with 1 and 5 being the least and most severe artifact, respectively. In the first-round evaluation, 54 videos without correction were graded. Then, 5 datasets from each grade level were picked to perform a second-round evaluation of three reconstructions (no correction, LF correction, and ES correction), where 75 videos were prepared and presented to two readers in a randomized order.
Results
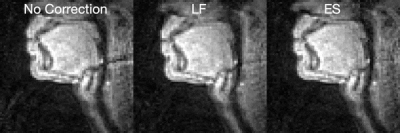
Figure 2 shows the simulation results, where the origin of the artifact is demonstrated. Figure 3 shows the artifact reduction results of one dataset. In the first-round evaluation, 46 datasets were found to have spiral aliasing artifact (Likert score ≥2 by at least one reader), resulted in 79% artifact rate. In the second-round evaluation, an average quantitative score improvement of 1.25 and 1.35 was observed for the LF and ES correction, respectively. Figure 4 shows the evaluation results. Reconstruction time increased by 228.8% (LF) and 6.9% (ES) compared with no correction. Table 1 concludes the qualitative score improvements and the reconstruction time.Discussion and Conclusion
We have identified the source of a common artifact observed in mid-sagittal spiral speech MRI. Simulations indicate the artifacts can be explained by gradient nonlinearity and inefficient readout low-pass filtering. We demonstrate two methods to correct this artifact: the LF and the ES methods. Both methods were found capable of reducing the artifact, with the ES method being more effective and more time efficient. These methods may be generally applied to spiral imaging where the desired FOV is smaller than the signal producing region.Acknowledgements
This work was supported by NSF Grant 1514544 and NIH Grant R01-DC007124. We acknowledge the Speech Production and Articulation kNowledge (SPAN) group at the University of Southern California for helpful discussions and collaboration.References
1. Doran SJ, Charles-Edwards L, Reinsberg SA, Leach MO. A complete distortion correction for MR images: I. Gradient warp correction. Phys Med Biol 2005;50(7):1343-1361.
2. Janke A, Zhao H, Cowin GJ, Galloway GJ, Doddrell DM. Use of spherical harmonic deconvolution methods to compensate for nonlinear gradient effects on MRI images. Magn Reson Med 2004;52(1):115-122.
3. Lee JH, Scott GC, Pauly JM, Nishimura DG. Broadband multicoil imaging using multiple demodulation hardware: a feasibility study. Magn Reson Med 2005;54(3):669-676.
4. Lazarus C, März M, Weiss P. Correcting the Side Effects of ADC Filtering in MR Image Reconstruction. Journal of Mathematical Imaging and Vision 2020;62(6):1034-1047.
5. Segars WP, Sturgeon G, Mendonca S, Grimes J, Tsui BM. 4D XCAT phantom for multimodality imaging research. Med Phys 2010;37(9):4902-4915.
6. Lingala SG, Toutios A, Töger J, Lim Y, Zhu Y, Kim Y-C, Vaz C, Narayanan SS, Nayak KS. State-of-the-Art MRI Protocol for Comprehensive Assessment of Vocal Tract Structure and Function. 2016; San Francisco, USA. p 475-479.
7. Lingala SG, Zhu Y, Kim YC, Toutios A, Narayanan S, Nayak KS. A fast and flexible MRI system for the study of dynamic vocal tract shaping. Magn Reson Med 2017;77(1):112-125.
8. Santos JM, Wright GA, Pauly JM. Flexible real-time magnetic resonance imaging framework. Conf Proc IEEE Eng Med Biol Soc 2004;2004:1048-1051.
9. Kerr AB, Pauly JM, Hu BS, Li KC, Hardy CJ, Meyer CH, Macovski A, Nishimura DG. Real-time interactive MRI on a conventional scanner. Magn Reson Med 1997;38(3):355-367.
10. Adluru G, Awate SP, Tasdizen T, Whitaker RT, Dibella EV. Temporally constrained reconstruction of dynamic cardiac perfusion MRI. Magn Reson Med 2007;57(6):1027-1036.
Figures