3518
Clinical Application of Twelve-fold Accelerated Submillimeter Whole Brain 3D-T2 weighted Imaging with Deep Learning Reconstruction
Yasutaka Fushimi1, Satoshi Nakajima1, Akihiko Sakata1, Takuya Hinoda1, Sonoko Oshima1, Sayo Otani1, Krishna Pandu Wicaksono1, Hiroshi Tagawa1, Yang Wang1, Masahiro Nambu2, Rimika Imai2, Koji Fujimoto3, Hitomi Numamoto4, Kanae Miyake4, Tsuneo Saga4, and Yuji Nakamoto1
1Kyoto University Hospital, Kyoto, Japan, 2MRI Systems Division, Canon Medical Systems Corporation, Otawara, Japan, 33. Department of Real World Data Research and Development, Kyoto University Graduate School of Medicine, Kyoto, Japan, 4Department of Advanced Medical Imaging Research, Kyoto University Graduate School of Medicine, Kyoto, Japan
1Kyoto University Hospital, Kyoto, Japan, 2MRI Systems Division, Canon Medical Systems Corporation, Otawara, Japan, 33. Department of Real World Data Research and Development, Kyoto University Graduate School of Medicine, Kyoto, Japan, 4Department of Advanced Medical Imaging Research, Kyoto University Graduate School of Medicine, Kyoto, Japan
Synopsis
Twelve-fold accelerated submillimeter 3D-T2 weighted imaging with DLR and imaging quality and sharpness of cranial nerves were examined.
INTRODUCTION
MR cisternography visualize the relationship between cranial nerves, intracranial vasculatures and cerebrospinal fluid (CSF) space. The use of MR cisternography is usually performed when some neurovascular compression syndrome and local cisternal abnormalities are clinically suspected. However, focused MR cisternography is frequently performed, meanwhile whole brain high resolution MR cisternography is rarely performed due to the limitation of scan time.Two-dimensional parallel imaging has been applied for 3D MR imaging, and but image noise associated with high acceleration factor degrades the image quality of the image sequence. Recently developed deep learning reconstruction (DLR) is expected to reduce noise with keeping imaging quality1,2. In addition, since the denoising is performed in consideration of the g-factor distribution, it is possible to adaptively remove the spatially non-uniform noise caused by parallel imaging. Therefore, effective denoising is expected even for high-speed imaging with a high g-factor.
In this study, we applied twelve-fold accelerated submillimeter 3D-T2 weighted imaging with DLR and imaging quality and sharpness of cranial nerves were examined.
METHODS
SubjectsLocal institutional committee approved this study. Twenty-three patients were enrolled in this prospective observation study. Written informed consent was obtained. All patients underwent 3D T2-weighted imaging (Fast Advanced Spin Echo, FASE, an equivalent sequence of half Fourier single-shot turbo spine echo, HASTE) at MR unit (Galan ZGO, Canon Medical Systems Corporation, Otawara, Japan) with 32-channel head coil.
MR image sequence
3D FASE: TR/TE, 1500/297.5 ms; constant flip angle, 89°; slice thickness, 0.7 mm, bandwidth 326 Px/Hz; field of view, 200 × 200 mm; matrix 704 × 704; resolution, 0.28 × 0.28 mm; acceleration factor of PE and SE, 3 × 4; sagittal acquisition; scan time, 1 min 27 sec.
Post-imaging process
- Contrast
noise ratio
ROIs placed on the lateral ventricles and pons (Figure 1). Contrast noise ratio of each structure was evaluated by mean value divided by standard deviation. - Sharpness of oculomotor nerves
RESULTS
Representative cases were shown in Figure 2. Image noise was evident in 3D-T2WI 12× without DLR, meanwhile, most of image noise was removed in 3D-T2WI 12× with DLR.- Contrast
noise ratio
CNR of pons and lateral ventricle is shown in Figure 3. Both CNR is significantly higher in 3D-T2WI with DLR compared with 3D-T2WI without DLR (P<0.001). - Sharpness
of the oculomotor nerves
Sharpness of oculomotor nerves was shown in Figure 4. No significant difference was shown in sharpness of oculomotor nerves between 3D-T2WI without DLR and that with DLR (P=0.63).
DISCUSSION
We have demonstrated 12-fold accelerated submillimeter whole brain 3D-T2WI with DLR showed better contrast noise ratio compared with 3D-T2WI without DLR. Scan time of around 1.5 min is clinically feasible and the delineation of fine structure is preserved after DLR processing.Visualization of finer structures such as arachnoid membrane is beneficial in neurosurgery, however, there are trade-offs between image resolution, imaging noise, and scan time. We believe higher resolution 3D-T2WI with DLR may help us solve such trade-off problems.
Sharpness of the oculomotor nerves were not different between two images. Image noise may affect the visualization of cranial nerves, however, the information of edge may be retained. Limitations. First, the limited number of patients were included in this study. Further studies are required to validate the clinical implication of 3D T2WI with high acceleration factor. Second, the image quality of other structures has not been investigated well by radiologists.
CONCLUSION
Twelve-fold accelerated submillimeter whole brain 3D T2WI with DLR showed better image quality compared with that without DLR, and the delineation of fine structure such as the oculomotor nerves was preserved after DLR processing.Acknowledgements
No acknowledgement found.References
1. Kidoh M, Shinoda K, Kitajima M, et al. Deep Learning Based Noise Reduction for Brain MR Imaging: Tests on Phantoms and Healthy Volunteers. Magn Reson Med Sci. 2020 Aug 3;19(3):195-206. doi: 10.2463/mrms.mp.2019-0018.
2. Sagawa H, Fushimi Y, Nakajima S, et al. Deep Learning-based Noise Reduction for Fast Volume Diffusion Tensor Imaging: Assessing the Noise Reduction Effect and Reliability of Diffusion Metrics. Magn Reson Med Sci. 2020 Sep 18. doi: 10.2463/mrms.tn.2020-0061. Online ahead of print.
Figures
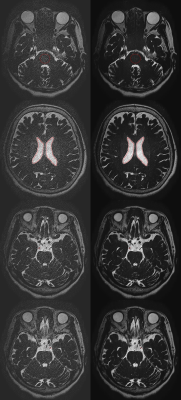
Figure 1. Whole
brain 3D-T2WI 12× without DLR (left
column) and whole brain 3D-T2WI 12× with
DLR (right column) are shown.
ROIs
placed on the pons (upper row) and the lateral ventricles (second row). A line
was placed over bilateral oculomotor nerves (third and bottom row) to calculate
sharpness of oculomotor nerves.
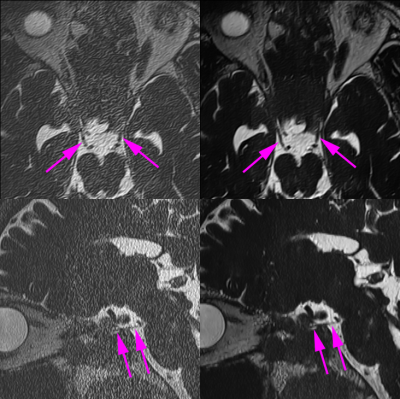
Figure 2. 3D-T2WI 12× (left column) and 3D-T2WI 12× with DLR (right column) are shown. Axial reconstructed images showed
bilateral oculomotor nerves (arrows). Oblique sagittal reconstructed images
showed right oculomotor nerve (arrows). Image noise was evident in 3D-T2WI 12× without DLR, meanwhile, most of image noise was removed in 3D-T2WI 12× with DLR.
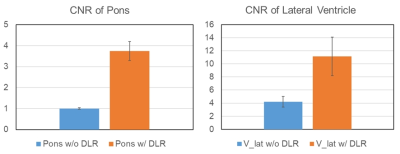
Figure 3. CNR
of pons and lateral ventricle is shown in Figure 3. Both CNR is significantly
higher in 3D-T2WI with DLR compared with 3D-T2WI without DLR (P<0.001).
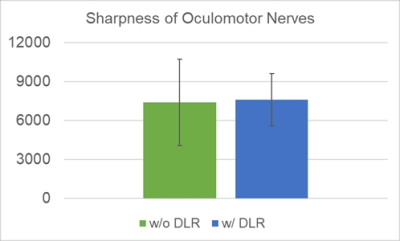
Figure 4. Sharpness
of oculomotor nerves was shown. No significant difference was shown in
sharpness of oculomotor nerves between 3D-T2WI without DLR and that with DLR
(P=0.63).