3511
Novel machine learning method for clinically significant cortical lesion detection in multiple sclerosis
Eve L Kazarian1, Mariam S Aboian1, and John D Port2
1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Radiology, Mayo Clinic, Rochester, MN, United States
1Department of Radiology and Biomedical Imaging, Yale University, New Haven, CT, United States, 2Department of Radiology, Mayo Clinic, Rochester, MN, United States
Synopsis
A novel tree-based machine learning model was applied to head MR imaging scans from multiple sclerosis (MS) patients in order to detect cortical gray matter lesions. Cortical lesions are strong indicators of MS disability yet are not easily visualized in clinical practice. The model was useful for accurately detecting cortical lesion presence (AUC of 0.78 ± 0.02).
Purpose
Multiple sclerosis (MS) is a CNS inflammatory demyelinating disease and is the most common cause of neurologic disability in young adults. While MS is considered a white matter disease, cortical gray matter lesions have been demonstrated to be the strongest predictor of MS disability. We propose a novel method for automatic detection of cortical lesions on advanced MR sequences.Materials and Methods
Clinical/imaging records of N=62 multiple sclerosis patients scanned at Mayo Clinic between January, 2014 and April, 2016 were reviewed, and N=50 subjects identified with cortical lesions. Volumetric 1 mm3 scans using MPRAGE, PSIR, GMDIR, and WMDIR were performed on each subject. Image volumes were processed using Freesurfer. First, the PSIR, GMDIR and WMDIR volumes were co-registered to the MPRAGE volume. Freesurfer then calculated the pial and gray-white junction (GWJ) surfaces which were manually cleaned up to improve accuracy. Surfaces parallel to the pial and GWJ surfaces were interpolated at a distance of1/3 and 2/3 the way between the pial and GWJ surfaces. We also interpolated a surface in the white matter approximately 1.3 mm deep to the GWJ surface (juxtacortical white matter) (Figure 1). A 17-element feature vector was constructed for each Freesurfer node containing the average image intensity from the four layers for each of the 4 contrasts, plus the cortical thickness at that node. Cortical lesion presence was manually determined by 4 human raters; lesion detection differences were adjudicated amongst the readers to arrive at “the truth.” Lesion presence/absence data were added to create a 21-element feature vector. Finally, an XGBoost machine learning model was applied for dichotomized classification of the presence of cortical lesions. A correlation and ranking of feature importance was performed at each validation of the model, removing highly correlated features for accurate representation of importance (Figure 2).Results
A total of 519 cortical lesions were identified in our N=50 subjects, corresponding to a total of 169,054 Freesurfer nodes with lesions out of a possible 12,769,300 (1.3%). The XGBoost model was useful for detecting cortical lesions (AUC of 0.78 ± 0.02). The model effectively distinguished between true positives and negatives in the dataset, with average sensitivity across all model iterations being 0.87 and specificity 0.93. Juxtacoritcal node intensities from the GMDIR, MPRAGE, and WMDIR sequences were most predictive for cortical lesion presence (Figure 3). Leukocortical lesions are the most common lesion type identified on MRI in clinical practice, which is consistent with our results that suggest that presence of juxtacortical signal abnormality indicates extension of abnormal signal extending into the adjacent inner cortex (a leukocortical lesion). Fitting the model on the same dataset without juxtacortical node intensities resulted in significantly diminished accuracy (AUC of 0.59 ± 0.10), further demonstrating the predictive power of juxtacortical measurements. Thickness of the lesion was also a high predictive feature in our model.Conclusions
We demonstrate a novel machine learning model for prediction of cortical demyelinating lesions that are not easily visualized in clinical practice. Such a model may assist neuroradiologists in clinical practice, increasing the accuracy of cortical lesion detection.Acknowledgements
Research was supported by Mayo Clinic’s Department of Neurology and Yale University's Department of Radiology and Biomedical Imaging.References
- Rudick RA, Trapp BD. Gray-matter injury in multiple sclerosis. N Engl J Med. 2009 Oct 8;361(15):1505-6. doi: 10.1056/NEJMcibr0905482. PMID: 19812410.
- Calabrese M, Agosta F, Rinaldi F, Mattisi I, Grossi P, Favaretto A, Atzori M, Bernardi V, Barachino L, Rinaldi L, Perini P, Gallo P, Filippi M. Cortical lesions and atrophy associated with cognitive impairment in relapsing-remitting multiple sclerosis. Arch Neurol. 2009 Sep;66(9):1144-50. doi: 10.1001/archneurol.2009.174. PMID: 19752305.
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. The New England journal of medicine 2000; 343:938-952.
- Chen, T., & Guestrin, C. XGBoost. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. 2016. doi: 10.1145/2939672.2939785
Figures
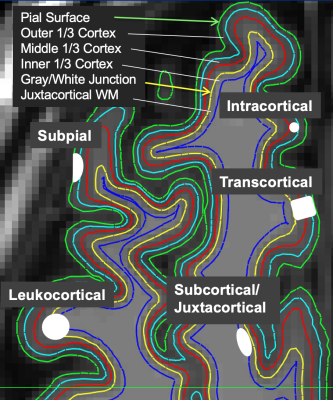
Figure 1: Subpial lesions are usually found in outer and middle cortical layers, transcortical lesions in all three cortical layers, intracortical in middle cortical layer, leukocortical in middle and outer cortical layers and juxtacortical white matter, and juxtacortical in the juxtacortical white matter.
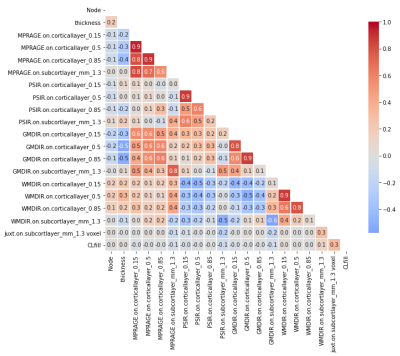
Figure 2: Correlation across features. Highly correlated features (MPRAGE.on.corticallayer_0.15, MPRAGE.on.corticallayer_0.5, PSIR.on.corticallayer_0.15, GMDIR.on.corticallayer_0.15, GMDIR.on.corticallayer_0.5, WMDIR.on.corticallayer_0.15) were removed for analysis.
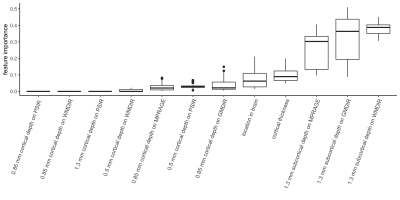
Figure 3: Predictive power across features.