3509
Parkinson’s Disease Subtype Identification Using Radiomics Based on Iron Deposition in Substantia Nigra1Department of Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Shanghai Key Laboratory of Magnetic Resonance, East China Normal University, Shanghai, China, 3Magnetic Resonance Innovations, Inc., Bingham Farms, MI, United States, 4Department of Radiology, Wayne State University, Detroit, MI, United States, 5Department of Biomedical Engineering, Wayne State University, Detroit, MI, United States
Synopsis
A total of 104 Parkinson’s disease (PD) patients and 269 age- and sex-matched healthy controls (HCs) were scanned using 3D multi-echo gradient echo MTC sequence. In this work, a data-driven clustering approach based on iron deposition in the substantia nigra (SN) measured by quantitative susceptibility mapping (QSM) was performed to classify the PD patients into different subtypes. The clinical assessments were compared between subtype groups. Two subtypes were found by using this clustering approach. Furthermore, there are significant differences (p-values < 0.05) on MDS-UPDRS scores between these two subtype groups.
Introduction
Previous studies suggested that patients with Parkinson’s disease (PD) can be classified into several subtypes1-3, but their identity and pathophysiological basis remain poorly understood. In PD, iron elevation in the substantia nigra (SN) is a major pathologic feature4. The increase of iron deposition in the SN is related to disease duration and severity5. Data-driven clustering approaches provide an unbiased method to detect groups of patients with similar profiles, thus may serve as a promising way for PD subtype classification. Our goal was to identify subtypes of PD using cluster analysis based on iron deposition in the SN measured by quantitative susceptibility mapping (QSM) and to compare the clinical assessments between different PD subtypes.Methods
A total of 104 PD patients (age range:29-81 years old) and 269 age- and sex-matched HCs (age range:31-89 years old) (cohort 1) were imaged on a 3T Philips Ingenia scanner using a 3D multi-echo gradient echo MTC sequence. The imaging parameters were as follows: TE1 = 7.5ms, ΔTE = 7.5ms, TR = 62ms, flip angle = 30˚, pixel bandwidth = 174Hz/pixel, matrix size = 384 × 384, slice thickness = 2mm, original spatial in-plane resolution = 0.67 × 1.34mm2 then interpolated to 0.67 × 0.67mm2. The second echo (TE = 15ms) was used for the QSM reconstruction to evaluate iron deposition in the SN. The susceptibility maps were created using the following steps: the brain extraction tool, BET, to isolate the brain tissue6, a 3D phase unwrapping algorithm (3DSRNCP) to unwrap the original phase data7, sophisticated harmonic artifact reduction (SHARP) to remove unwanted background fields8, and a truncated k-space division (TKD) based inverse filtering technique9 with an iterative approach to reconstruct the final QSM maps10. For this data driven approach, we used the manual tracings of the SN from 165 QSM images to train 3D U-Net11 to perform automatic segmentation of the SN. The trained artificial neural network was tested on 19 cases. All 184 cases (Cohort 2:165 cases for training and 19 cases for testing, both from a previous unpublished work) were healthy controls and were collected using the same imaging protocol described above. This algorithm was then used to segment the 104 PD cases and 269 HCs mentioned earlier (Cohort 1). After we finished the SN segmentation, 107 radiomics features were extracted from each SN ROI. We used the radiomics features from the 269 HCs to construct a standard feature model and to eventually determine the abnormal features of the PD patients. The distance between the observed PD features and the extracted radiomics features from the HCs can be used to determine the probability of the features being abnormal. The detailed modelling steps can be summarized as: 1) To remove redundant features, the 107 radiomics features were clustered into 10 groups. In each group, features which strongly correlated with clinical scores (by Pearson correlation analysis) were selected12. 2) Radiomics features from 269 HCs were used to train a Gaussian process regressor, expected radiomics feature values can be predicted for the PD patients. The abnormal probability feature Z was defined as: Z=Δ(YPD, Y'PD)/δ, where and denote the observed and expected feature value of PD cases, respectively. Δ(,) and δ indicate distance function and Gaussian standard error. 3) K-Means clustering was used to obtain the final PD subtypes. We used random forest embedding to map the features to a sparse representation, and then used multidimensional scaling approach to reduce the feature dimensions. For clinical validation, a t-test was used for the comparison of MOCA and UPDRS scores between clustered subtypes. The overall framework of our approach is illustrated in Fig. 1.Results
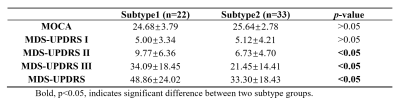
As for SN segmentation, we achieved an average Dice-coefficient of 0.95 based on the test data. Two PD subtypes were obtained using K-Means clustering, denoted as Subtype1 and Subtype2, which included 50 and 54 PD cases respectively. The clustering result is shown in Fig. 2. Fifty-five PD cases with clinical scores available (a sub-cohort of PD cases in Cohort 1) were used for the subtype groups comparison (Table 1). Significant differences for MDS-UPDRS Ⅱ, Ⅲ and total score were found between the two subtypes (p-values < 0.05).Discussion and Conclusion
In this work, two PD subtypes were identified using a data-driven approach based on iron deposition features in the SN. Significant differences of UPDRS scores were found between these two PD subtype groups. The iron deposition in SN based on QSM is a reflection of the PD pathophysiology and it could provide a strong unbiased classification biomarker for PD. In the future, the longitudinal evaluation of the iron deposition and the disease progression with a larger sample size would help validate the subtype classification of our approach.Acknowledgements
NoneReferences
1. Wang, L., Cheng, W., Rolls, E. T., Dai, F., Gong, W., Du, J., ... & Brown, P. (2020). Association of specific biotypes in patients with Parkinson disease and disease progression. Neurology, 95(11), e1445-e1460.
2. De Pablo-Fern´andez E, Lees AJ, Holton JL, Warner TT. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol 2019;76: 470–479.
3. Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB. Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 2017; 140:1959–1976.
4. Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003; 24:197–211.
5. Bergsland, N., Zivadinov, R., Schweser, F., Hagemeier, J., Lichter, D., & Guttuso Jr, T. (2019). Ventral posterior substantia nigra iron increases over 3 years in Parkinson's disease. Movement Disorders, 34(7), 1006-1013.
6. Smith SM. Fast robust automated brain extraction. Human brain mapping. 2002; 17(3): 143-55.
7. Abdul-Rahman HS, Gdeisat MA, Burton DR, et al. Fast and robust three-dimensional best path phase unwrapping algorithm. Applied optics. 2007; 46(26): 6623-35.
8. Schweser F, Deistung A, Lehr BW, et al. Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage. 2011; 54(4): 2789-807.
9. Haacke EM, Tang J, Neelavalli J, et al. Susceptibility mapping as a means to visualize veins and quantify oxygen saturation. Journal of magnetic resonance imaging: JMRI. 2010; 32(3): 663-76.
10. Tang J, Liu S, Neelavalli J, et al. Improving susceptibility mapping using a threshold-based K-space/image domain iterative reconstruction approach. Magnetic resonance in medicine: official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2013; 69(5): 1396-407.
11. Çiçek, Ö., Abdulkadir, A., Lienkamp, S. S., Brox, T., & Ronneberger, O. (2016, October). 3D U-Net: learning dense volumetric segmentation from sparse annotation. In International conference on medical image computing and computer-assisted intervention (pp. 424-432). Springer, Cham.
12. Fiset S, Welch ML, Weiss J, Pintilie M, Conway JL, Milosevic M, Fyles A, Traverso A, Jaffray D, Metser U, Xie J. Repeatability and reproducibility of MRI-based radiomic features in cervical cancer. Radiotherapy and Oncology. 2019 Jun 1; 135:107-14.
Figures