3488
Preoperative MR Radiomics and ADC Value for Prediction of Progression and Recurrence in Meningiomas1Department of Medical Imaging, Chi Mei Medical Center, Tainan, Taiwan, 2Department of Health and Nutrition, Chia Nan University of Pharmacy and Science, Tainan, Taiwan, 3Department of Radiological Sciences, University of California, Irvine, CA, CA, United States, 4Department of Radiology, E-Da Hospital and I-Shou University, Kaohsiung, Taiwan
Synopsis
A subset of meningiomas may show early progression/recurrence (P/R) after surgery. In clinical practice, one of the main challenges in the treatment of meningiomas is to determine factors that correlate with P/R. This study investigated the role of preoperative MR radiomics and apparent diffusion coefficient (ADC) value for prediction of P/R in meningiomas. The four most significant radiomic features selected by support vector machine (SVM) were used to calculate SVM score for each patient. High SVM score and low ADC value were associated with early P/R and short progression-free survival in meningiomas.
Background and Purpose
Meningiomas are the most common commonly diagnosed primary brain tumors [1]. Although most meningiomas are classified as benign tumors according to the 2016 WHO classification system [2], a subset of these tumors may show early progression/ recurrence (P/R) after surgical resection [3,4]. Further, the rate of P/R is relatively high in tumors difficulty in achieving gross total resection, such as for parasagittal and skull base meningiomas [5,6]. The conventional MR imaging findings such as tumor size, skull bone invasion, and invasion into major sinuses had been reported as the important variables related to P/R [3,6]. However, analysis of quantitative preoperative MR radiomics and apparent diffusion coefficient (ADC) value for prediction of P/R in meningiomas is still rare. In this study, we investigated the role of preoperative MR radiomics based on support vector machine (SVM) and ADC value for the prediction of P/R in meningiomas.Materials and Methods
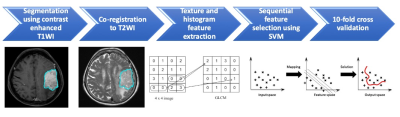
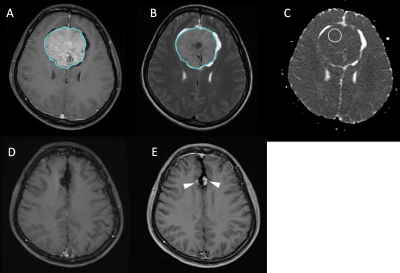
128 patients (age 26 - 84 years; median age, 57.5 years) diagnosed with WHO grade I meningiomas by MRI and pathological confirmation were included. The median follow-up time was 64 months (range 14 - 149 months). A total of 19 (19/128, 14.8%) patients were found to have P/R, and 107 patients remained stable disease. The MRI images were acquired using a 1.5T or a 3.0T scanner. The protocols of MR imaging included axial and sagittal spin echo T1-weighted imaging (T1WI), fast spin-echo T2-weighted imaging (T2WI), contrast-enhanced (CE) T1WI in axial and coronal sections, and diffusion weighted imaging (DWI). The DWI was performed by applying sequentially in the x, y, and z direction with b =1000 sec/mm2, and ADC maps were obtained from these imaging data. For ADC measurement, a circular ROI (area ranging from 35 to 78 mm2) was placed within the tumor area. Figure 1 shows the flowchart of the analysis process. The tumor was segmented from CE T1WI images. Then the outline of the lesion ROI on each imaging slice was automatically obtained using the fuzzy c-means (FCM) clustering based algorithm [7]. The ROIs from all imaging slices containing this lesion were combined to obtain 3D information of the whole lesion. Then 3D connected-component labeling was applied to remove scattered voxels not connecting to the main lesion ROI, and hole-filling to include all voxels contained within the main ROI which were labeled as non-lesion. The segmented tumor mask was co-registered to T2W images to localize the tumor location on corresponding images using affine transformation. This process was done by FMRIB’s Linear Image Registration Tool (FLIRT) [8]. Within segmented tumor on CE T1WI and T2W images , 32 first order features and 75 textural features were extracted on each modality. Thus, totally we obtained 214 radiomic features. To evaluate the importance of these features in differentiating P/R, sequential feature selection process was utilized via constructing multiple SVM classifiers. Four features with the highest importance, including T1 GLCM cluster shade, T1 GLSZM grey level non-uniformity, T2 GLCM cluster prominence, and T2 GLCM cluster shade were selected by the final SVM classification model with Gaussian kernel [9]. 10 folds cross-validation method was applied to test the model performance [10]. The personalized SVM score was calculated for each patient based on the four selected features as described below. This procedure was implemented in MATLAB 2018b.Results
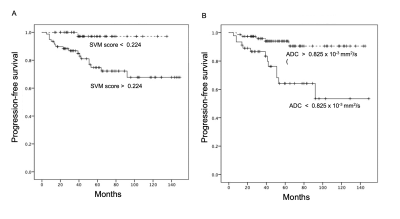
Nineteen (19/128, 14.8%) patients were diagnosed with P/R after surgery. The most significant four imagining features selected by the SVM model for prediction of P/R were T1 GLCM cluster shade, T1 GLSZM grey level non-uniformity, T2 GLCM cluster prominence, and T2 GLCM cluster shade. Statistically significant differences (p < 0.05) were observed in the extent of resection, adjacent bone invasion, ADC value, and SVM score between P/R and non-P/R groups (Figure 2). In multivariate Cox hazards analysis, adjacent skull bone invasion, low ADC value, and high SVM score were high risk factors for P/R (p < 0.05) with hazard ratios of 7.31, 4.67, and 8.13 respectively. For prediction of P/R, AUCs of 0.80 and 0.73 with optimal cut-off values of 0.224 and 0.825 x 10-3 mm2/s were obtained in SVM score and ADC value respectively (Figure 3). Improved performance in predictive model was observed after combining SVM score and ADC value, with AUC of 0.88 (Figure 3). When tumor progression trends were compared, patients with higher SVM score and lower ADC value were found to exhibit shorter progression-free survival (PFS) (p < 0.05) (Figure 4).Conclusions
This study attempted to use preoperative MR radiomics and ADC value for prediction of P/R in meningiomas. High personalized SVM score and low ADC value were associated with early P/R and short PFS in meningiomas. The results offer useful information for treatment planning in meningiomas.Acknowledgements
NoneReferences
1. Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99 (3):307-314. doi:10.1007/s11060-010-0386-3.
2. Louis DN, Perry A, Reifenberger G, Von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta neuropathologica 131 (6):803-820.
3. Ildan F, Erman T, Gocer AI, Tuna M, Bagdatoglu H, Cetinalp E, Burgut R (2007) Predicting the probability of meningioma recurrence in the preoperative and early postoperative period: a multivariate analysis in the midterm follow-up. Skull base : official journal of North American Skull Base Society [et al] 17 (3):157-171. doi:10.1055/s-2007-970554
4. Maillo A, Orfao A, Espinosa AB, Sayagues JM, Merino M, Sousa P, Lara M, Tabernero MD (2007) Early recurrences in histologically benign/grade I meningiomas are associated with large tumors and coexistence of monosomy 14 and del(1p36) in the ancestral tumor cell clone. Neuro Oncol 9 (4):438-446. doi:10.1215/15228517-2007-026
5. Savardekar A, Patra D, Bir S, Thakur J, Mohammed N, Bollam P, Georgescu M, Nanda A (2017) Differential tumor progression patterns in skull base versus non-skull base meningiomas: A critical analysis from a long-term follow-up study and review of literature. World neurosurgery 112:e74-e83. doi: 10.1016/j.wneu.2017.12.035. Epub 2017 Dec 16.
6. Escribano Mesa JA, Alonso Morillejo E, Parron Carreno T, Huete Allut A, Narro Donate JM, Mendez Roman P, Contreras Jimenez A, Pedrero Garcia F, Masegosa Gonzalez J (2018) Risk of Recurrence in Operated Parasagittal Meningiomas: A Logistic Binary Regression Model. World neurosurgery 110:e112-e118. doi:10.1016/j.wneu.2017.10.087.
7. Nasrabadi NM (2007) Pattern recognition and machine learning. Journal of electronic imaging 16 (4):049901.
8. Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17 (2):825-841.
9. Tong S, Chang E (2001) Support vector machine active learning for image retrieval. Proceedings of the ninth ACM international conference on Multimedia: p. 107-118.
10. Friedman J, Hastie T, Tibshirani R (2001) The elements of statistical learning: Springer series in statistics New York, NY, USA.
Figures