3479
Diffusion Tensor Imaging of the Roots of the Brachial Plexus: A Systematic Review and Meta-Analysis of Normative Values1University of Leeds, Leeds, United Kingdom, 2Sheffield Teaching Hospitals, Sheffield, United Kingdom, 3Umeå University, Umeå, Sweden, 4University of Pittsburgh, Pittsburgh, PA, United States
Synopsis
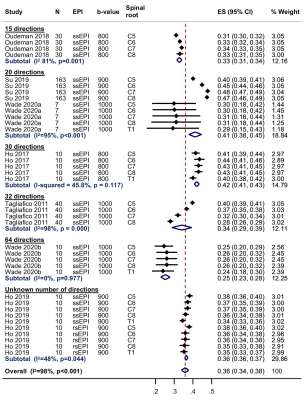
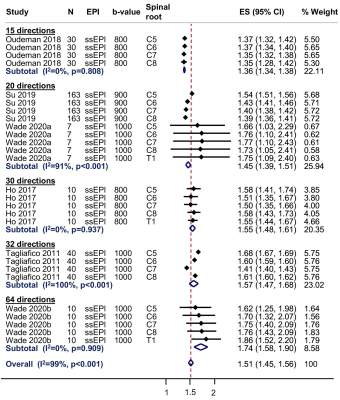
In this systematic review and meta-analysis we summarise the normal diffusion tensor imaging (DTI) metrics for roots of the brachial plexus. We included 9 studies describing 316 adults (1:1 male:female) of mean age 35 years (SD 6). The normal fractional anisotropy was 0.36 (95% CI 0.34, 0.38; Figure 4). The normal mean diffusivity was 1.51 x10-3 mm2/s (95% CI 1.45, 1.56; Figure 5). DTI metrics varied according to experimental conditions and participant factors. Our summary estimates from different conditions which may be valuable to researchers and clinicians alike.
Introduction
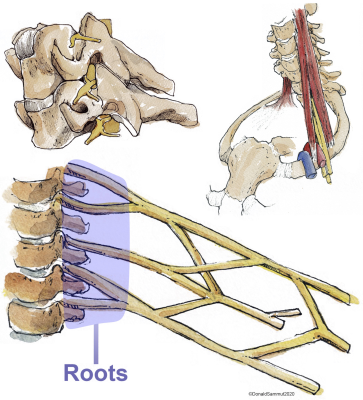
Magnetic resonance imaging (MRI) is the best non-invasive imaging modality for diagnosing various pathologies affecting the brachial plexus (Figure 1)[1–6]. The roots of the brachial plexus are the most common site of injury[7] and typically, the status of the root dictates the prognosis and surgical reconstruction. However, the diagnostic performance of conventional cross-sectional MRI for assessing the roots remains suboptimal[7] and consequently, there has been a recent surge of research into diffusion tensor imaging (DTI), which characterises tissue microstructure and provides proxy measures of myelination, axon diameter, fibre density and organisation. Before DTI can be used clinically to assess the ‘health’ of the roots of the brachial plexus, there is a need to estimate the normal values.Methods
This review is registered with PROPSERO (ID CRD42019155788), it was designed and conducted in accordance with the Cochrane Handbook of Systematic Reviews[8] and has been authored in accordance with the PRISMA checklist[9]. We systematically searched the literature for studies of asymptomatic adults who underwent DTI of the brachial plexus. Participant characteristics, scanning protocols, and measurements of the fractional anisotropy (FA) and mean diffusivity (MD) of each spinal root were extracted by two independent review authors. Generalised linear modelling was used to estimate the effect of experimental conditions on the FA and MD. Meta-analysis of root-level estimates were performed using Cohen’s method with random-effects.Results
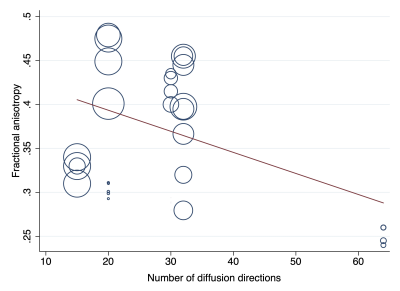
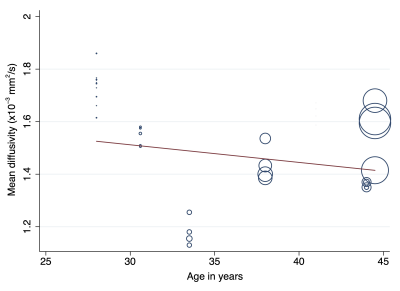
After reviewing 27 full texts, 15 were excluded. We included 9 studies[10–18], describing 316 adults (1:1 male:female) of mean age 35 years (SD 6) were included. Multivariable modelling showed that the angular resolution was strongly associated with FA, whereby every additional 10 diffusion sensitising gradient directions sampled reduced the FA by 0.01 (95% CI 0.01, 0.03; Figure 2). Furthermore, multivariable modelling showed that each year of life reduced the MD by 0.03 x10-3 mm2/s (95% CI 0.01, 0.04; Figure 3). The pooled normal fractional anisotropy of the roots of the brachial plexus at 3 Tesla[10,11,13,16–19] was 0.36 (95% CI 0.34, 0.38; Figure 4). There were no statistically significant differences between the C5-T1 roots. The pooled normal mean diffusivity of the roots of the brachial plexus at 3 Tesla [11,13,16–19] was 1.51 x10-3 mm2/s (95% CI 1.45, 1.56; Figure 5).Discussion
We have shown that the roots of the brachial plexus in adults have a mean fractional anisotropy of 0.36 (95% CI 0.34, 0.38) and mean diffusivity of 1.51 x10-3 mm2/s (95% CI 1.45, 1.56). However, there is substantial variation between studies and the estimates are shown to be sensitive to experimental factors[20], such as: the SNR[21], components of the b-value[22,23], the number of diffusion sensitising gradient directions[24,25]; software pipelines for denoising and correcting artefacts arising from susceptibility, motion or eddy-currents[26,27], tensor fitting methods[21], and the size and position of ROIs[28]. Whilst the TraCED challenge[29] and several phantom studies[30–32] have demonstrated that very high reproducibility across scanners, sequences and sessions for DTI, we suggest that researchers and clinicians interpret our summary values with respect to their particular circumstances.Conclusions
The FA and MD of the roots of the brachial plexus vary according to experimental conditions and participant factors. We provide summary estimates of the normative values in different conditions which may be valuable to researchers and clinicians alike.Acknowledgements
No acknowledgement found.References
1. Vargas MI, Viallon M, Nguyen D, Beaulieu JY, Delavelle J, Becker M. New approaches in imaging of the brachial plexus. Eur J Radiol. Elsevier Ireland Ltd; 2010;74:403–10.
2. Nardin R a, Patel MR, Gudas TF, Rutkove SB, Raynor EM. Electromyography and magnetic resonance imaging in the evaluation of radiculopathy. Muscle Nerve. 1999;22:151–5.
3. Zhu Y-S, Mu N-N, Zheng M-J, Zhang Y-C, Feng H, Cong R, et al. High-Resolution Ultrasonography for the Diagnosis of Brachial Plexus Root Lesions. Ultrasound Med Biol. 2014;40:1420–6.
4. Mallouhi A, Meirer R, Bodner G. Sonographic features of brachial plexus traumatic rupture. J Neurosurg; 2003;99:432–432.
5. Lapegue F, Faruch-Bilfeld M, Demondion X, Apredoaei C, Bayol MA, Artico H, et al. Ultrasonography of the brachial plexus, normal appearance and practical applications. Diagn Interv Imaging. France: Elsevier Masson SAS; 2014;95:259–75.
6. Sureka J, Cherian RA, Alexander M, Thomas BP. MRI of brachial plexopathies. Clin Radiol. 2009;64:208–18.
7. Wade RG, Takwoingi Y, Wormald JCR, Ridgway JP, Tanner S, Rankine JJ, et al. MRI for Detecting Root Avulsions in Traumatic Adult Brachial Plexus Injuries: A Systematic Review and Meta-Analysis of Diagnostic Accuracy. Radiology. 2019;293:125–33.
8. Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. Cochrane Collab. 2011;
9. Moher D, Liberati A, Tetzlaff J, Altman DG. Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annu Intern Med. 2009;151:264–9.
10. Ho MJ, Ciritsis A, Manoliu A, Stieltjes B, Marcon M, Andreisek G, et al. Diffusion Tensor Imaging of the Brachial Plexus: A Comparison between Readout-segmented and Conventional Single-shot Echo-planar Imaging. Magn Reson Med Sci. 2019;18:150–7.
11. Ho MJ, Manoliu A, Kuhn FP, Stieltjes B, Klarhöfer M, Feiweier T, et al. Evaluation of Reproducibility of Diffusion Tensor Imaging in the Brachial Plexus at 3.0 T. Invest Radiol. 2017;52:482–7.
12. Chen M, Li X, Chen J, Sun C, He J. Quantitative analysis of the normal brachial plexus with diffusion tensor imaging. Chinese J. Med. Imaging Technol. X.-C. Li, Department of Radiology, The First Affiliated Hospital of Guangzhou Medical College, Guangzhou 510120, China. E-mail: xinchunli@163.com: Editorial Board of Chinese Journal of Medical Imaging Techno (PO Box 2712, Beijing 100080, China); 2012. p. 77–81.
13. Oudeman J, Verhamme C, Engbersen MP, Caan MWA, Maas M, Froeling M, et al. Diffusion tensor MRI of the healthy brachial plexus. Gelderblom M, editor. PLoS One. (Oudeman, Engbersen, Caan, Maas, Nederveen) Department of Radiology, Academic Medical Center, Amsterdam, Netherlands: Public Library of Science (E-mail: plos@plos.org); 2018;13:e0196975.
14. Su X, Kong X, Liu D, Kong X, Alwalid O, Wang J, et al. Multimodal magnetic resonance imaging of peripheral nerves: Establishment and validation of brachial and lumbosacral plexi measurements in 163 healthy subjects. Eur J Radiol. Elsevier; 2019;117:41–8.
15. Vargas MI, Viallon M, Nguyen D, Delavelle J, Becker M. Diffusion tensor imaging (DTI) and tractography of the brachial plexus: feasibility and initial experience in neoplastic conditions. Neuroradiology. 2010;52:237–45.
16. Wade RG, Teh I, Andersson G, Fang-Cheng Y, Wiberg M, Bourke G. Diffusion Tensor Imaging of the Brachial Plexus: Defining the Fractional Anisotropy Threshold for Deterministic Tractography of the Roots. FigShare Prepr. 2020;
17. Wade RG, Tanner SF, Teh I, Ridgway JP, Shelley D, Chaka B, et al. Diffusion Tensor Imaging for Diagnosing Root Avulsions in Traumatic Adult Brachial Plexus Injuries: A Proof-of-Concept Study. Front Surg. 2020;7:Article 19.
18. Tagliafico A, Calabrese M, Puntoni M, Pace D, Baio G, Neumaier CE, et al. Brachial plexus MR imaging: accuracy and reproducibility of DTI-derived measurements and fibre tractography at 3.0-T. Eur Radiol.; 2011;21:1764–71.
19. Samardzic M, Rasulic L, Grujicic D, Bacetic D, Milicic B. Nerve transfers using collateral branches of the brachial plexus as donors in patients with upper palsy-thirty years’ experience. Acta Neurochir (Wien). (Samardzic, Rasulic, Grujicic) Neurosurgical Clinic, Clinical Center of Serbia, University of Belgrade, Belgrade, Serbia: Springer Wien (Sachsenplatz 4-6, P.O. Box 89, Vienna A-1201, Austria); 2011;153:2009–19.
20. Helmer KG, Chou M-C, Preciado RI, Gimi B, Rollins NK, Song A, et al. Multi-site study of diffusion metric variability: effects of site, vendor, field strength, and echo time on regions-of-interest and histogram-bin analyses. In: Gimi B, Krol A, editors. Physiol Behav. 2016. p. 97882U.
21. Jones DK, Basser PJ. “Squashing peanuts and smashing pumpkins”: How noise distorts diffusion-weighted MR data. Magn Reson Med. 2004;52:979–93.
22. Schilling KG, Nath V, Blaber J, Harrigan RL, Ding Z, Anderson AW, et al. Effects of b-value and number of gradient directions on diffusion MRI measures obtained with Q-ball imaging. Styner MA, Angelini ED, editors. Proc SPIE Int Soc Opt Eng. 2017;101330N.
23. Qin W, Shui Yu C, Zhang F, Du XY, Jiang H, Xia Yan Y, et al. Effects of echo time on diffusion quantification of brain white matter at 1.5T and 3.0T. Magn Reson Med. 2009;61:755–60.
24. Haakma W, Pedersen M, Froeling M, Uhrenholt L, Leemans A, Boel LWT. Diffusion tensor imaging of peripheral nerves in non-fixed post-mortem subjects. Forensic Sci Int. Elsevier Ireland Ltd; 2016;263:139–46.
25. Giannelli M, Cosottini M, Michelassi MC, Lazzarotti G, Belmonte G, Bartolozzi C, et al. Dependence of brain DTI maps of fractional anisotropy and mean diffusivity on the number of diffusion weighting directions. J Appl Clin Med Phys. 2010;11:176–90.
26. Taylor PA, Alhamud A, van der Kouwe A, Saleh MG, Laughton B, Meintjes E. Assessing the performance of different DTI motion correction strategies in the presence of EPI distortion correction. Hum Brain Mapp. 2016;37:4405–24.
27. Haddad SMH, Scott CJM, Ozzoude M, Holmes MF, Arnott SR, Nanayakkara ND, et al. Comparison of quality control methods for automated diffusion tensor imaging analysis pipelines. Yap P-T, editor. PLoS One. 2019;14:e0226715.
28. Vos SB, Jones DK, Viergever MA, Leemans A. Partial volume effect as a hidden covariate in DTI analyses. Neuroimage. Elsevier Inc.; 2011;55:1566–76.
29. Nath V, Schilling KG, Parvathaneni P, Huo Y, Blaber JA, Hainline AE, et al. Tractography reproducibility challenge with empirical data (TraCED): The 2017 ISMRM diffusion study group challenge. J Magn Reson Imaging. 2020;51:234–49.
30. Vavasour IM, Meyers SM, Mädler B, Harris T, Fu E, Li DKB, et al. Multicenter Measurements of T 1 Relaxation and Diffusion Tensor Imaging: Intra and Intersite Reproducibility. J Neuroimaging. 2019;29:42–51.
31. Prohl AK, Scherrer B, Tomas-Fernandez X, Filip-Dhima R, Kapur K, Velasco-Annis C, et al. Reproducibility of Structural and Diffusion Tensor Imaging in the TACERN Multi-Center Study. Front Integr Neurosci. 2019;13:1–15.
32. Kimura M, Yabuuchi H, Matsumoto R, Kobayashi K, Yamashita Y, Nagatomo K, et al. The reproducibility of measurements using a standardization phantom for the evaluation of fractional anisotropy (FA) derived from diffusion tensor imaging (DTI). Magn Reson Mater Physics, Biol Med. Springer International Publishing; 2019;15–9.
Figures