3456
Single-Shot Inner-Field-of-View Fast Spin Echo Imaging with MAVRIC: Access to the Spinal Cord Close to Metallic Implants11Department of Systems Neuroscience, University Medical Center Hamburg–Eppendorf, Hamburg, Germany
Synopsis
Inner field-of-view EPI provides a good image quality for diffusion-weighted imaging of the spinal cord in healthy subjects but suffers from severe artifacts in the vicinity of metallic implants that may be present in patients with traumatic injuries. Here, inner-field-of-view imaging based on cross-sectional RF pulses is used to obtain a single-shot fast spin echo technique that combined with multi acquisition variable-resonance imaging (MAVRIC) and view angle tilt (VAT) offers a more robust access to small target regions close to metallic implants and, thus, may be feasible for diffusion-weighted imaging of patients with traumatic spinal cord injury.
Introduction
Inner field-of-view EPI, e.g. (1,2), provides a good image quality for diffusion-weighted imaging (DWI) of the spinal cord in healthy subjects. But it suffers from severe artifacts in the vicinity of metallic implants making it unfeasible for most patients with traumatic injuries. Fast-spin-echo (FSE) imaging, in particular when combined with multi acquisition variable-resonance imaging (MAVRIC)(3) and view angle tilt (VAT)(4) is much more robust, however, may not be applicable in a single-shot as desired for DWI. Here, inner-field-of-view imaging based on cross-sectional RF pulses is combined with FSE imaging to enable single-shot imaging that in combination MAVRIC and VAT can provide a much better image quality close to metallic objects than EPI and, thus, may be feasible for diffusion-weighted imaging of patients with traumatic spinal cord injury.Materials and Methods
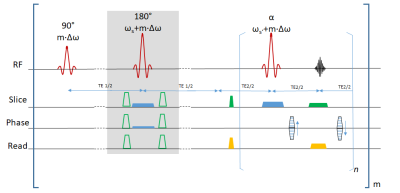
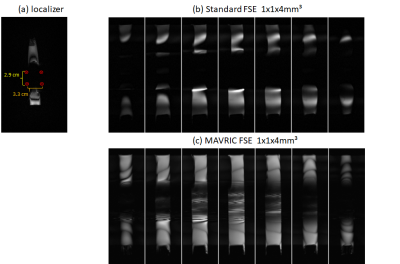
The basic pulse sequence (Fig. 1) has been derived from a standard fast-spin-echo sequence. An extra refocusing section is inserted prior to the readout interval that could be used to apply diffusion weighting and is tilted by an angle ɸ compared to the image plane. The latter allows to reduce the FOV in the phase-encoding direction without aliasing and can shorten the echo train accordingly to make it feasible for single-shot acquisitions. To introduce the frequency-selectivity required for MAVRIC, the initial RF excitation is applied without slice-selection gradient pulse but different frequency offsets that differ between subsequent acquisitions of the same slice; the same frequency offsets are also considered for all subsequent slice-selective RF pulses and add to the frequency required for the conventional slice selection. The readout is performed with view angle tilt, i,e. a gradient pulse in the slice direction during the data acquisition. Measurements were performed on a 3T whole-body MR system (Siemens PrismaFit) using a 64 channel head-neck coil and a 32 channel spine coil on phantoms with metallic objects (four aluminum screws; see Fig. 2) and a volunteer with a metallic implant from whom informed consent was obtained prior to the examination. A resolution of 1.0x1.0x4.0mm³ covering a FOV of 32x128 mm² was used yielding an echo spacing of 7.4ms, an echo time of 35ms for an ascending sampling for the FSE. With a frequency width of the RF pulses of about 1000Hz, the MAVRIC frequency range between +10kHz and -10kHz for phantom measurements and 2000 Hz and -2000Hz in vivo was sampled in steps of 500Hz in an interleaved order. All frequencies were stepped through for a slice with a TR of 900ms yielding a total acquisition time per slice of 1.13min for phantom and of 8.1s for in vivo measurements before continuing with the next slice. A maximum intensity projection across frequency offsets was used to calculate a single image for each slice on the fly. The EPI measurement yielded a total measurement duration of 5 s with a TR of 4200 ms and a TE of 65 ms.Results and Discussion
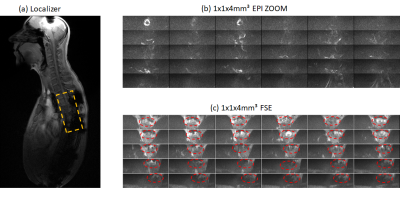
Figure 2 summarizes results obtained in the phantom with metallic objects (four aluminum screws). Signal losses close to and even at a distance from the screws are obvious in the localizer image (Fig. 2a) and within the FSE acquisitions without MAVRIC (Fig. 2b). With MAVRIC, significant signal can be regained and almost the full phantom becomes visible, although with some residual interferences and remaining signal losses. These images underline the significant influence of even smaller metallic objects and the capability of the MAVRIC approach to recover signal. In figure 3 measurements of a volunteer with metallic implants are presented. Thereby the cancellations due to the implants are apparent in the localizer (a). Further in Fig 3 transversal in vivo measurements with inner FOV EPI (b) and inner FOV FSE (c) are shown. In case of the EPI images the spinal cord is nearly not identifiable, the distortions due to the metallic screws and plates are too strong. With the combination of FSE and MAVRIC the spinal cord can be observed and in nearly all slices parts of it are recognizable, even though the SNR is rather low. The image quality difference in between FSE and EPI is due to the different refocusing techniques used, RF refocused and gradient refocused, respectively, as the least reacts more prone to inhomogeneities. The reason for the varying measured frequency ranges during the phantom and in vivo acquisitions are due to the different susceptibilities of the implant materials. The susceptibility of aluminum, as used for the phantom, is by a factor of 10² larger compared to titanium as present in the volunteer and thus leads to stronger distortions.Conclusion
The combination of FSE with ZOOM and MAVRIC proves to be a tool with which the image acquisition near metallic artifacts can be improved significantly. Still work has to be done to accelerate the acquisition time. A possibility to accelerate the image acquisition could lie in combination of MAVRIC with simultaneous multi-slice imaging.Acknowledgements
This work was supported by a grant of Wings for LifeReferences
[1] Saritas EU. DWI of the spinal cord with reduced FOV single-shot EPI. MRM, 2008;60: 468-473
[2] Wheeler-Kingshott CA. ADC mapping of the human optic nerve: Increased resolution, coverage and reliability with csf-suppressed ZOOM-EPI, MRM, 2002 (47) 24-31
[3] Koch KM et al., Imaging near metal with a MAVRIC-SEMAC hybrid. Magn Reson Med, 2011 (65) 71-82
[4] Cho ZH et al Total inhomogeneity correction including chemical shifts and susceptibility by view angle tilting, A. A. Phys. In Medicine 1988, (15), 7-11
Figures