3454
Spinal Cord Compression is Associated with Brain Plasticity in Degenerative Cervical Myelopathy1Medical Biophysics, University of Western Ontario, London, ON, Canada, 2Centre for Functional and Metabolic Mapping, Robarts Research Institute, London, ON, Canada, 3Clinical Neurological Sciences, University Hospital, London Health Sciences Centre, London, ON, Canada
Synopsis
Degenerative cervical myelopathy (DCM) is one of the most common forms of spinal cord dysfunction. Predicting functional recovery after surgery remains elusive. The extent of cortical activation in the primary motor cortex was assessed using fMRI when DCM patients performed a controlled finger-tapping task. Spine compression severity was quantified using T2-weighted imaging. Patients with severe spine compression showed larger activation volumes. Recruitment of neurons to compensate for functional deficits may explain these activation changes. Hypoxia at the spine compression site may drive this cortical plasticity. Future studies should measure hypoxia and explore prognostic determinants.
Introduction
Degenerative cervical myelopathy (DCM) is one of the most common forms of spinal cord dysfunction, with the incidence and prevalence in North America estimated to be 41 and 605 per million, respectively.1 It is a unique model of spinal cord injury that can result in compression of the spinal cord2 and lead to neurological dysfunction.3 Surgical intervention can effectively prevent the progression of neurological decline and improve functional outcome; however, some patients continue to deteriorate and predicting surgical outcome has proven difficult. Patient demographic factors such as age4, level(s) of compression5, or duration of symptoms6 have all been proven as unreliable prognostic determinates. Because the pathophysiological mechanisms of the disease are only partially understood, predicting a patient’s potential for functional recovery following surgical intervention remains elusive. To improve the prognostic determinates of DCM, we must better understand the relationship between localized compression in the cord, neuronal damage, and cortical reorganization. We hypothesized that cortical reorganization would be greater in patients with more severe compression, indicating neurological injury in the spinal cord.Methods
A 3.0 T Siemens Prisma Fit MRI scanner was used to acquire functional images of the brain in 23 patients (14 men, mean age (± SD) 65 ± 14.5 years) with symptoms of DCM and no other neurological disorders. These images were acquired using an interleaved echo planar imaging pulse sequence (2.3 mm isotropic resolution, TR/TE = 1000/30 msec, matrix size = 720 x 720, flip angle = 40˚). With their right hand, patients were instructed to perform a structured finger-tapping task to activate the motor cortex to assess the extent of cortical activation. This was also repeated for the left hand. Volume of activation (VOA) and % blood oxygen level-dependent (BOLD) signal were determined using region-of-interest analysis. T2-weighted images of the spine were also acquired using a spin-echo pulse sequence (0.90 mm isotropic resolution, TR/TE = 2170/135 msec, matrix size = 256 x 256, 2 averages). They were used to quantify the severity of spinal cord compression and total compression volume was found by using the semi-automatic segmentation software, Spinal Cord Toolbox7, with segmentation shown in Figure 1. Using in-house MATLAB code, the total volume of the spinal cord in the compressed region was measured. To measure the reliability of the spinal cord compression measurement, two raters performed repeated measurements of cord compression on the randomized dataset three separate times and the intraclass correlation (ICC) was used to determine the intra- and inter-rater reliability.Results
Measurements of the spinal cord compression volume were highly reproducible, with the reliability between the two raters characterized with an ICC of 0.977. The intra-rater reliability of each rater was also substantial, with the first rater achieving an ICC of 0.996 and the second rater achieving an ICC of 0.967. The observed BOLD signal increase in the contralateral primary motor cortex was associated with increased spinal cord compression severity when patients tapped with their left hand (r = 0.49, p = 0.02) and right hand (r = 0.56, p = 0.005), as shown in Figure 2. The VOA in the contralateral primary motor cortex also increased with compression severity when patients tapped with their left hand (r = 0.55, p = 0.006) and right hand (r = 0.45, p = 0.03), as shown in Figure 3.Discussion
DCM patients with severe spinal cord compression recruit larger regions of the motor cortex to perform finger-tapping tasks. This expansion of cortical activity when performing the controlled motor task may be related to cortical plasticity and the rewiring of the axons of the lower limb extremities into the hand region8 to compensate for the difficulty with the instructed task. This significant correlation is consistent with the presence of pathophysiological changes occurring in the spine, like ischemia and hypoxia, and it is reasonable to hypothesize that the observed cortical reorganization is a compensatory mechanism to neurological injury in the spinal cord.Conclusion
The current study demonstrates that DCM patients recruit larger regions of the primary motor cortex to tap their fingers when spinal cord compression is more severe, which is consistent with pathophysiological changes occurring in the spine. These pathophysiological changes could impact recovery following surgery; however, direct in-vivo evidence has been limited in humans. Future studies measuring hypoxia and/or ischemia at the site of compression may provide more insight into the mechanisms of the neurological injury and help us further understand the prognostic determinates of DCM patients.Acknowledgements
The authors thank all participants for their contribution to this project. We also thank Scott Charlton and Oksana Opalevych (CFMM, Robarts Research Institute, The University of Western Ontario) for facilitating MRI acquisitions.References
[1] Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative Cervical Myelopathy: Epidemiology, Genetics, and Pathogenesis. Spine 2015; 40: E675-693.
[2] Toledano M, Bartleson JD. (2013). Cervical spondylotic myelopathy. Neurol Clin 2013; 31: 287-305.
[3] Nurick S. The natural history and the results of surgical treatment of the spinal cord disorder associated with cervical spondylosis. Brain 1972; 95: 101-108.
[4] Lu J, Wu X, Li Y, Kong X. (2008). Surgical results of anterior corpectomy in the aged patients with cervical myelopathy. Eur Spine J 2008; 17: 129–135.
[5] Fessler RG, Steck JC, Giovanini MA. Anterior cervical corpectomy for cervical spondylotic myelopathy. J Neurosurg 1998; 43: 257–267.
[6] Yamazaki T, Yanaka K, Sato H, Uemura K, Tsukada A, Nose T. Cervical spondylotic myelopathy: surgical results and factors affecting outcome with special reference to age differences. J Neurosurg 2003; 52: 122-126.
[7] De Leener B, Lévy S, Dupont SM, Fonov VS, Stikov N, Collins DL, Callot V, et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage 2017; 145: 24–43.
[8] Ghosh A, Haiss F, Sydekum E, Schneider R, Gullo M, Wyss MT, et al. Rewiring of hindlimb corticospinal neurons after spinal cord injury. Nat Neurosci 2010; 13: 97–104.
Figures
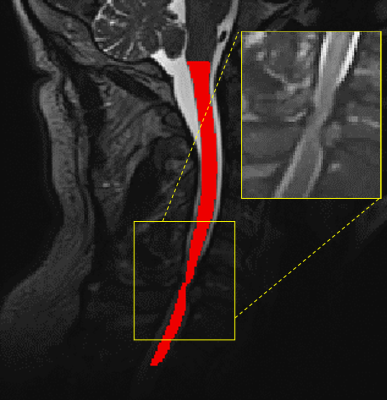
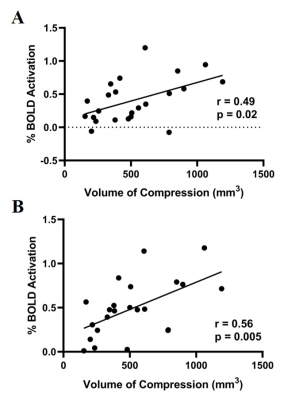
Figure 2: A: % BOLD signal correlation with spinal cord compression volume when tapping with left hand. B: % BOLD signal correlation with spinal cord compression volume when tapping with right hand.
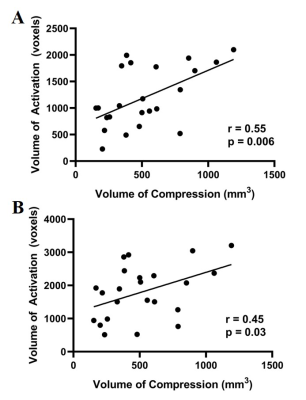
Figure 3: A: Volume of activation correlation with spinal cord compression volume when tapping with left hand. B: Volume of activation correlation with spinal cord compression volume when tapping with right hand.