3446
Free Water Eliminated White Matter Tract Integrity of Spinal Cord in Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder
Masaaki Hori1,2, Kouhei Kamiya1,2, Akifumi Hagiwara2,3, Kazumasa Yokoyama4, Issei Fukunaga5, Katsuhiro Sano2, Koji Kamagata2, Katsutoshi Murata6, Shohei Fujita2, Christina Andica2, Akihiko Wada2, Julien Cohen-Adad7, and Shigeki Aoki2
1Radiology, Toho University Omori Medical Center, Tokyo, Japan, 2Radiology, Juntendo University School of Medicine, Tokyo, Japan, 3Radiology, David Geffen School of Medicine, Los Angeles, CA, United States, 4Neurology, Juntendo University School of Medicine, Tokyo, Japan, 5Juntendo University School of Medicine, Tokyo, Japan, 6Siemens Japan K.K, Tokyo, Japan, 7NeuroPoly Lab, Polytechnique Montreal, Montréal, QC, Canada
1Radiology, Toho University Omori Medical Center, Tokyo, Japan, 2Radiology, Juntendo University School of Medicine, Tokyo, Japan, 3Radiology, David Geffen School of Medicine, Los Angeles, CA, United States, 4Neurology, Juntendo University School of Medicine, Tokyo, Japan, 5Juntendo University School of Medicine, Tokyo, Japan, 6Siemens Japan K.K, Tokyo, Japan, 7NeuroPoly Lab, Polytechnique Montreal, Montréal, QC, Canada
Synopsis
We investigated free water eliminated kurtosis-based white matter tract integrity to distinguish microstructural changes in the spinal cords of patients with MS and Neuromyelitis Optica. FA was significant higher in spinal cord white matter in MS (P=0.0025). There was no significant difference in other diffusion model-based metrics. The values of FA seem to be non-specific but robust. Therefore, clinical feasible and more optimized diffusion microstructural models and data acquisitions for spinal cord may be needed to provide an additional information and to be biomarker in patients with MS and NMOSD in vivo.
Introduction:
Multiple sclerosis (MS) and Neuromyelitis Optica Spectrum Disorder (NMOSD) are diseases of immune system attack and they lead to disability from nervous system damage. In the past, some NMOSD had been misdiagnosed and treated as a form of MS. Nowadays, scientific consensus distinguishes MS and NMOSD because the pathologic processes and effective treatments are different. However, the values of conventional MR imaging is limited for structural changes and demonstration of insufficient lesions1, and the evaluation of so-called normal-appearing damaged tissue method remains to be established for both diseases. As recently showed, diffusion kurtosis imaging-derived white matter tract integrity (WMTI) metrics2, 3 might provide more pathological specificity and clinically meaningful information in brain of MS patients4. However, partial volume effects with the surrounding cerebral spinal fluid are biasing diffusion MRI measurements in the spinal cord. Recently, Free water elimination technique was introduced to correct for free water bias in diffusion MRI5. The purpose of this study is to investigate the impact of free water elimination in WMTI metrics to distinguish microstructural change in the spinal cords of MS and NMOSD patients.Methods:
In this prospective study, we enrolled 35 MS patients (age 47±11 years, 26 females; 31 RRMS, 4 SPMS, median EDSS score 2.0) and 18 NMOSD patients (age 57±17 years, 15 females). After conventional MR imaging including T2-, T2*- and T1-wegted imaging, 2-shell diffusion MR imaging data using regional excitation technique (ZoomIt) were acquired with a Siemens Prisma 3T scanner with a body coil excitation and 64-ch head/neck coil for reception. Imaging parameters for 2-shell dMRI were as follows: repetition time (TR)/echo time: 2200/76 (ms/ms); section thickness: 5 mm; 39 slices; in-plane pixel size: 0.9x 0.9 mm; SMS factor: 2; imaging time: approximately 12 min; 2 b values (1000 and 2000 s/mm 2) with two b=0 images and diffusion encoding in 30 direction for every b value. All diffusion MRI data were transferred to an offline workstation and processed using in-house developed software in Matlab (R2019a, Math Works, Inc, Natick, MA). After free water elimination model5 was applied for all the diffusion MRI data, parametric maps of mean diffusivity (MD), mean kurtosis (MK), fractional anisotropy (FA), axonal water fraction (AWF), axial and radial intra-axonal diffusivities (Di, axial and Di, radial, respectively) and axial and radial extra-axonal diffusivities (De, axial and De, radial, respectively)4 were calculated. We refer to the branches as Branch 1 (yielding Da ≤ De,∥) and Branch 2 (yielding Da > De,∥), as Hansen et al6. Moreover, semi-automated analysis was performed using the Spinal Cord Toolbox7 for segmentation, motion correction, registration to WM atlas and extraction of metrics (Figure 1). Between MS and NMOSD, quantitative metrics in the white matter at C2, C3, C4 and C5 were selected and compared. Statistical evaluations were performed by using R version 3.5.0 (http://www.r-project.org) using t- test. we calculated the false discovery rate (FDR) using the Benjamini-Hochberg method to ac-count for multiple hypothesis tests. P value less than 0.05 was considered statistically significant.Results:
All metrics values of white matter of spinal cords in patients with MS and NMOSD were summarized in table 1 and Figure 2. Only FA at C5 spinal level was significant higher in spinal cord white matter in patients with MS (P=0.0025), after FDR adjustment. There was no difference in the other metrics.Discussion:
Our results show that the diffusion metric values seem to be reasonable, but only FA seems to be useful in capturing the different pathological microstructural change in the spinal cord white matter in MS and NMOSD, presumably caused by different degrees of demyelination and axonal damages. KG Schilling et al. pointed out that the signal model such as diffusion tensor, which makes no explicit assumptions on microstructure, showed strong correlations with all ground truth indices of spinal cord microstructure8. The values of FA seem to be non-specific but sensitive and robust. Therefore, clinically feasible and more optimized diffusion microstructural models and data acquisitions for spinal cord may be needed to provide an additional information and to be biomarker in patients with MS and NMOSD.Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 19K08161, the Canada Research Chair in Quantitative Magnetic Resonance Imaging [950-230815], the Canadian Institute of Health Research [CIHR FDN-143263], the Canada Foundation for Innovation [32454, 34824], the Fonds de Recherche du Québec - Santé [28826], the Fonds de Recherche du Québec - Nature et Technologies [2015-PR-182754], the Natural Sciences and Engineering Research Council of Canada [435897-2013], the Canada First Research Excellence Fund (IVADO and TransMedTech) and the Quebec BioImaging Network [5886].References
- Miki Y, et al.Relapsing-remitting multiple sclerosis: longitudinal analysis of MR images--lack of correlation between changes in T2 lesion volume and clinical findings. Radiology. 1999 ;213(2):395-9.
- Fieremans et al. White matter characterization with diffusional kurtosis imaging. Neuroimage. 2011 ;58(1):177-88.
- Veraart J, et al. More accurate estimation of diffusion tensor parameters using diffusion Kurtosis imaging. Magn Reson Med. 2011 ;65(1):138-45.
- de Kouchkovsky I, et al. Quantification of normal-appearing white matter tract integrity in multiple sclerosis: a diffusion kurtosis imaging study. J Neurol. 2016 ;263(6):1146-55.
- A.R. Hoy et al.Optimization of a free water elimination two-compartment model for diffusion tensor imaging NeuroImage 2014; 103:323-333
- Hansen B, et al. White matter biomarkers from fast protocols using axially symmetric diffusion kurtosis imaging. NMR Biomed. 2017 ;30(9). doi: 10.1002/nbm.3741.
- De Leener B,et al. SCT: Spinal Cord Toolbox, an open-source software for processing spinal cord MRI data. Neuroimage. 2017 ;145(Pt A):24-43.
- K.G. Schilling et al. Diffusion MRI microstructural models in the cervical spinal cord –Application, normative values, and correlations with histological analysisNeuroImage 2019;201: 116026
Figures
Figure1. Representative
metric maps of a case with multiple sclerosis (41-year-old woman) and analysis
process.
Figure
2. The results of all metrics values of white matter of spinal cords at each
spinal level in patients with MS and NMOSD.
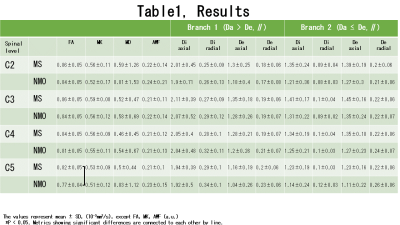
Table 1.
The results of all metrics values of white matter of spinal cords at each
spinal level in patients with MS and NMOSD.