3437
A novel clinically viable method to quantify T2 of intra and extra axonal compartmental tissue properties1Psychology, University of Pittsburgh, Pittsburgh, PA, United States, 2Nvidia, Bethesda, MD, United States, 3Mathematics, Indian Institute of Technology Kanpur, Kanpur, India
Synopsis
Diffusion MRI based microstructural imaging is used to estimate voxel wise intra/extra-cellular volume fraction and compartment diffusivities. Previously proposed techniques like NODDI, SMT models are used to estimate intra/extra-axonal volume fraction, axial and radial diffusivity for each voxel. TEdDI is a framework to estimate intra/extra-axonal T2 in human brain. The proposed TEdDI model requires multiple sets of diffusion dataset varies with echo time. In this study we are proposing SMT based formulation to estimate intra/extra-axonalT2. We also used this formulation to design numerical algorithm that approximate T2 and other parameters for a typical multi-shell diffusion acquisition.
Introduction
Microstructural imaging is used to quantify tissue properties using multi-shell diffusion imaging techniques1,2,3,4. Tissue can be modeled as three or two compartments: intra-axonal and extra-axonal (and free). Using multi-shell or diffusion spectrum imaging-based sampling, DW-MRI can be used to characterize the microarchitecture of biological tissue like white matter using popular diffusion modeling methods like CHARMED3, NODDI2, and SMT1.NODDI2, SMT1, and similar methods assume the same T2 decay for each compartment and can be truncated from the mathematical model by normalizing DWIs with non-diffusion MRI (b=0). Recently Veraart4 et al proposed a framework, TEdDI4, that can model T2, volume fraction, and axial/radial diffusivities for intra/extra-axonal compartment. Although TEdDI4 is a very comprehensive model with the caveat of requiring a scanning protocol with multiple sets of multi-shell diffusion imaging dataset varying echo-time for each set.
We propose a novel modeling framework that extends SMT1 to estimate T2 for Intra/extra-axonal compartments. Simultaneously, we also propose a numerical framework to estimate T2 for extra/intra-axonal compartments that utilize a typical multi-shell diffusion MRI protocol. The proposed method requires a different minimum of echo-time for each shell.
Method
Data acquisition: A healthy 38-year-old male was scanned on 3T Siemens TrioTim magnet with b=1000 s/mm2 and b=3000 s/mm2 with 64 gradient directions and b=5000 s/mm2 with 128 gradient directions along with b=0 images for every 10 of diffusion-weighted images with a total acquisition time of 17 minutes. Other MR parameters are as follows; FoV 230.4x230.4 mm: voxel size: 2.4x2.4x2.4mm3 with 63 axial slices. Minimum TE is chosen for each shell is chosen to be minimum, 92ms, 125ms, and 147ms for b=1000, 3000, and 5000 shells respectively.Preprocessing: Each diffusion shell was preprocessed separately and then registered together using b=0 images from each shell. Denoising8, unring9, N4 bias correction12, eddy10, and topup11 correction were performed using mrtrix, FSL6, and ANTS12 software packages. Preprocessed data was utilized to estimate the powered average for each shell. The mean value of DWIs was calculated using mrtrix and further used in SMT1 based formulation. Scipy’s curve_fit function is used to optimize for non-linear fitting for each voxel.
Formulation: We have used SMT1 to fit the estimated powered average MR signal for each that is dependent on b-value and TE. TE-dependent SMT model is described in the following equation:
$$\overline{S}(TE,b)=\rho[{\ f}_{in}{\ e}^{-\ \frac{TE}{T_2^{in}}}\ \psi(\ b\lambda\ )\ +\ {(1.0\ -\ f}_{in})\ {\ e}^{-\ \frac{TE}{T_2^{ex}}}\psi(\ bf_{in}\lambda)\ ]$$
where $$$ \psi(\ x)\ =\sqrt\pi\ erf(\sqrt x)/2x $$$ with $$${\lambda\ =\lambda}_\parallel=(1.0\ -\ f_{in})\ \lambda_\bot$$$ and $$$T_2^{in}$$$ and $$$T_2^{ex}$$$ are T2 values for intra/extra-axonal compartment. $$$f_{in}$$$ is the volume fraction for the intracellular compartment. $$$ \lambda_\parallel$$$ and $$$\lambda_\bot$$$are axial and radial diffusivity for intra/extra-axonal compartment respectively. The tortuosity condition is used for the final equation.
Numerical Optimization: Equation can be solved as a non-linear constrained optimization problem with four parameters {$$$f_{in}$$$, $$$\lambda$$$, $$$T_2^{in}$$$ and $$$T_2^{ex}$$$ }. It is an ill-posed problem and can have multiple local minima and hence does not converge optimally under all scenarios. To find an optimal solution, we first find an approximate solution, that can be used as an initial guess and then the interval around the approximate solution can be used as constrained to nonlinear optimization.
Here are steps for estimating an approximate solution:
- Estimate proton density,$$$\rho$$$ , for b=0 by assuming, $$$T_2^{in}\approx T_2^{ex}\approx T_2$$$,
2. Estimate $$$f_{in}$$$ and $$$\lambda$$$ using the following equation:
$$ \frac{\overline{S}(TE,\ b)}{\overline{S}(TE,\ b=0)}=[{\ f}_{in}\ \psi(\ b\lambda\ )\ +\ {(1.0\ -\ f}_{in})\ \psi(\ bf_{in}\lambda)\ ]$$
3. For large b-value, $$$b_{l} \approx 5000$$$ s/mm2, the extracellular compartment signal can be negligible5, and the equation1 can be reduced to one-compartment model and can be solved for $$$T^{in}_{2}$$$ using an estimated value for $$$\rho$$$, $$$f_{in}$$$ and $$$\lambda$$$,
$$ T_2^{in}=\frac{TE}{ln\left(\frac{{\ f}_{in}\ \psi(b_l\ \lambda)}{\frac{\overline{S}(TE,\ b_l)}{\rho}}\right)}$$
4. For small b-value, $$$b_{s} \approx 1000$$$ s/mm2, we can estimate $$$T_2^{ex}$$$ by substituting values estimated in steps 1-3.
$$ T_2^{ex}=\frac{TE}{ln\left(\frac{{(1.0\ -\ f}_{in})\ \psi(b_s\ \lambda{\ f}_{in})}{\frac{\overline{S}(TE,\ b_s)}{\rho}\ -\ {\ f}_{in}e^{-\frac{TE}{T_2^{in}}}\ \psi(\ b_s\lambda\ )}\right)}$$
Using the estimated values for $$$f_{in}$$$ and $$$\lambda$$$, $$$T_2^{in}$$$ and $$$T_2^{ex}$$$ as initial guess and interval around it (±30% for T2 and ±10% for $$$f_{in}$$$ and $$$\lambda$$$) as constraint, we can solve equation using linear least squares or Levenberg-Marquardt algorithm depending upon constraints. This was implemented using scipy’s curve_fit function.
Results and Discussion
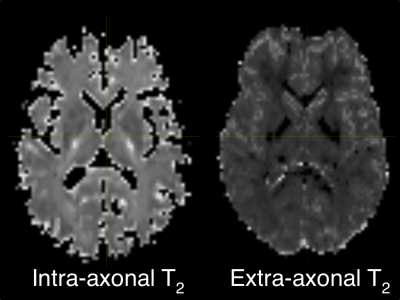
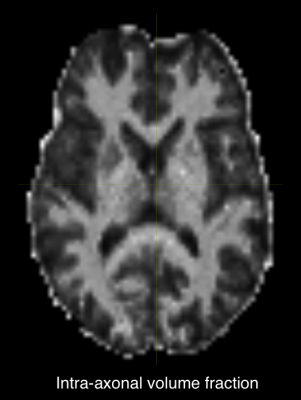
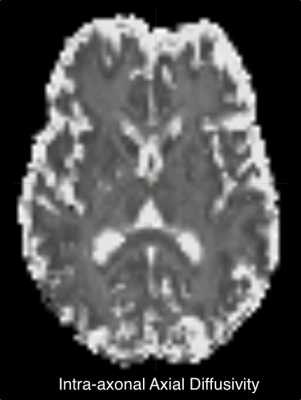
Fig.1 shows an axial slice of T2 values for intra/extra-axonal compartments. This slice looks similar to the estimated T2 in [1]. Intra-axonal T2 in the internal capsule shows high values which suggested highly myelinated axons in those regions. We report estimated average T2 for all white matter ROIs in the whole brain.Fig.2 shows the axial slice for the intra-axonal volume fraction. This shows a clear segmentation of white matter similar to typical SMT1 fit. Similarly, intra/extra-axonal diffusivity maps are shown in (Fig.3) indicating the expected values for each tissue type.
Conclusion
We have proposed an extended SMT framework to estimate intra/extra-axonal T2 along with volume fraction and axial and radial diffusivity. A novel numerical framework is also presented to estimate all parameters for a typical multi-shell diffusion protocol. T2 for Intra/extra-axonal compartment can be a potential biomarker for diseases like multiple-sclerosis or Alzheimer’s disease. In future studies will explore compartment T2 of brain-tissue damage in TBI-population.Acknowledgements
This project is supported in part by NIH/NINDS R44-NS103729 for diffusion phantom measurement, VA contract Quantitative Quality Assurance (VA-QQA) MRI for Brain Health NIH BRAIN Initiative, “Connectome 2.0: Developing the next generation human MRI scanner for bridging studies of the micro-, meso- and macro-connectome”, 1U01EB026996-01, and the David Scaife Foundation as part of supporting novel of brain tissue and phantom ground truth diffusion measurement.
References
- Kaden E, Kelm ND, Carson RP, Does MD, and Alexander DC: Multi-compartment microscopic diffusion imaging. NeuroImage, vol. 139, pp. 346–359, 2016
- Zhang, Hui, et al. "NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain." Neuroimage 61.4 (2012): 1000-1016
- Assaf, Yaniv, and Peter J. Basser. "Composite hindered and restricted model of diffusion (CHARMED) MR imaging of the human brain." Neuroimage 27.1 (2005): 48-58
- Veraart, Jelle, Dmitry S. Novikov, and Els Fieremans. "TE dependent Diffusion Imaging (TEdDI) distinguishes between compartmental T2 relaxation times." NeuroImage 182 (2018): 360-369.
- Li, Hua, et al. "Linking spherical mean diffusion weighted signal with intra-axonal volume fraction." Magnetic resonance imaging 57 (2019): 75-82.
- Jenkinson, Mark, et al. "Fsl." Neuroimage 62.2 (2012): 782-790.
- Tournier, J.-D.; Smith, R. E.; Raffelt, D.; Tabbara, R.; Dhollander, T.; Pietsch, M.; Christiaens, D.; Jeurissen, B.; Yeh, C.-H. & Connelly, A. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 2019, 202, 116137
- Veraart, J.; Fieremans, E. & Novikov, D.S. Diffusion MRI noise mapping using random matrix theory. Magn. Res. Med., 2016, 76(5), 1582-1593, doi: 10.1002/mrm.26059
- Kellner, E; Dhital, B; Kiselev, V.G & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magnetic Resonance in Medicine, 2016, 76, 1574–1581
- Andersson, J. L. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. NeuroImage, 2015, 125, 1063-1078
- Andersson, J. L.; Skare, S. & Ashburner, J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage, 2003, 20, 870-888
- Tustison, N.; Avants, B.; Cook, P.; Zheng, Y.; Egan, A.; Yushkevich, P. & Gee, J. N4ITK: Improved N3 Bias Correction. IEEE Transactions on Medical Imaging, 2010, 29, 1310-1320
Figures