3436
Time-dependent and TE-dependent Diffusivity in Human Brain using Multi-TE Oscillating Gradient Spin Echo in High Gradient 3.0T MRI (MAGNUS)1GE Global Research, Niskayuna, NY, United States
Synopsis
Oscillating gradient spin echo (OGSE) diffusion imaging achieves a short diffusion time and has been performed to assess tissue characteristics at short length scale, including the correlation length of the brain tissues which may provide information on axonal beading and swelling in disease. Time-dependent diffusivity has been measured from OGSE acquisition at a fixed TE in a high-performance head-only high-gradient 3.0T MRI scanner (MAGNUS). In this study, we characterized time-dependent diffusivity at varying frequencies (0~80 Hz) and TEs (72~158 ms) using multi-TE OGSE acquisition in healthy volunteers. Preliminary results showed increased mean/parallel diffusivities in corpus callosum at shorter TE.
INTRODUCTION
Oscillating gradient spin echo (OGSE) based diffusion imaging has shown great potential of characterizing the varying diffusivity at short diffusion time which reveals the length scale of brain microstructures including axon diameter1,2, correlation length of structural restrictions3-5. Assessing these microstructures provides information more specific to underlying pathology pathways of neurological disorders and diseases, including axonal beading and swelling5-7. Previous OGSE acquisitions in human subjects have showed increased diffusivities measurements at higher frequencies, equivalent to shorter diffusion times. A fixed TE is usually used and the diffusion-weighted signals are normalized by T2-weighted images at the same TE, with the hypothesis of a single T2 of the tissue. However, tissue compartments including intra-axonal and extra-axonal water have been shown to have different T2 relaxometry8,9. The T2 effects lead to different diffusivity measurements when TE varies. The characteristics of the time-dependent diffusivity at varying TEs have not yet been assessed. In this study, we propose to characterize the time-dependent diffusivity at varying TEs using multi-TE OGSE acquisitions.METHODS
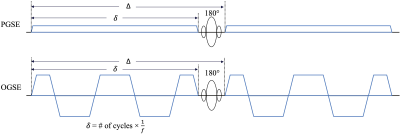
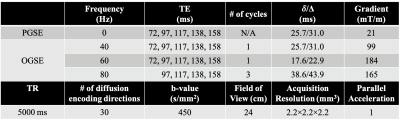
MRI Acquisition: In this IRB-approved study, one healthy volunteer were scanned in a 3.0T MRI system (MR750, GE Healthcare, WI) modified with a head-only MAGNUS gradient coil achieving 200 mT/m amplitude and 500 T/m/s slew rate for each gradient axis10, with a 32-channel phase-array head coil (Nova Medical, MA). OGSE cosine-modulated trapezoid waveforms11 were used for diffusion encoding (Figure 1). Additionally, conventional pulsed gradient spin echo (PGSE) with a flattened waveform12 was also acquired and considered to sample the 0 Hz diffusion spectrum. Imaging parameters are listed in Table 1. 25 slices covering the corpus callosum were obtained. The total scan time was 50 mins. T1-weighted MPRAGE anatomical structural images were also acquired.Image reconstruction: All OGSE and PGSE images were distortion corrected and registered to T1-weighted anatomical images. Mean/parallel/radial diffusivity (MD/PD/RD) and fractional anisotropy (FA) maps were calculated for each frequency at each TE. Different white matter parcels were segmented and identified using atlas.
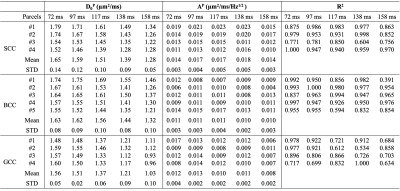
Data analysis: Mean values of MD, PD, RD and FA were calculated over the pixels in each parcel. The mean PD/RD of all frequencies at each TE was fitted to the 1-/2- dimensional short-range disorder model3,5, PD(f) = D0P + AP・f1/2and RD(f) = D0R + AR・f, respectively, where the intercept D0 correlates with the tortuosity, and the slope A correlates with the correlation length.
RESULTS
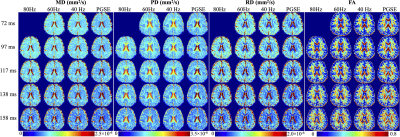
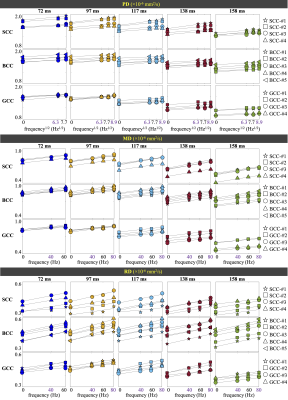
MD, PD, RD and FA maps at different TEs and different OGSE frequencies are shown in Figure 2. The averaged MD, PD and RD values of different parcels in the splenium (SCC), body (BCC), and genu (GCC) of the corpus collosum are shown in Figure 3. At a fixed TE, MD, PD, and RD measurements increase as the OGSE frequency increases. At a fixed frequency, MD, PD and RD are higher at shorter TEs, indicating the diffusivity measurements are TE-dependent.The functional dependence of PD and RD on OGSE frequencies fits the 1-/2- dimensional short-range disorder models (line in Figure 3), respectively. The fitted intercept (D0P), slope (AP) and goodness-of-fit R2 of PD are listed in Table 2.
PD and MD of parcels in SCC, BCC and GCC at a fixed TE and OGSE frequency in Figure 3 are shown to vary, indicating regional difference of the mesoscopic structure. The relative relationship between diffusivity measurements of two parcels is consistent when TE varies. For example, relatively higher diffusivity (e.g., SCC-#1 vs. SCC-#4) is maintained to be relatively higher when TE varies, even though the absolute measurements are changed as qualitatively compared in Figure 2. This trend is consistent with quantitative measurements of the PD intercept D0P in Table 2. However, the relative difference is different at different TEs, e.g., higher PD difference between SCC-#1 and SCC-#4 in SCC at TE of 72 ms, compared to the small PD difference at TE of 158 ms in Figure 2. Quantitatively, the standard deviation of sub-parcels’ PD intercept D0P in SCC in Table 2 is large at TE of 72 ms and small at TE of 158 ms, which is consistent with the qualitative assessment.
DISCUSSION AND CONCLUSION
We have characterized the diffusivity measurements of human corpus callosum at different TEs and OGSE frequencies. The high-performance MAGNUS gradient platform enables acquiring shorter TE and higher OGSE frequencies, compared to whole-body scanners. Both TE and diffusion time have shown to affect the diffusivity measurements. Thus, these two effects need to be accounted when comparing diffusivity measurements between acquisitions using different imaging parameters.The varying diffusivity measurements at different TEs indicate non-monoexponential T2 relaxation in the corpus callosum and other white matter bundles (not shown). Previous studies have shown a short T2 and a long T2 compartments in the white matter8,9. T2 effects of different compartments in a voxel have not been accounted for in the short-range disorder diffusion model. More importantly, the conventional diffusivity measurements may not be specific to a biophysical measurement. The multi-TE OGSE acquisition in this study provides two dimensions, relaxometry and diffusivity with varying TEs and diffusion times, to potentially disentangle different tissue compartments and assess individual microstructures. Future studies will be performed on modeling and quantification, to improve the accuracy of assessing brain tissue microstructures.
Acknowledgements
The authors acknowledge the funding support from U.S. Department of Defense, W81XWH-16-2-0054. The authors also thank Eric Fiveland for operating the MRI system.References
1. Xu J, Li H, Harkins KD, Jiang X, Xie J, Kang H, Does MD, Gore JC. Mapping mean axon diameter and axonal volume fraction by MRI using temporal diffusion spectroscopy. NeuroImage 2014;103:10-19.
2. Xu J, Li H, Li K, Harkins KD, Jiang X, Xie J, Kang H, Dortch RD, Anderson AW, Does MD, Gore JC. Fast and simplified mapping of mean axon diameter using temporal diffusion spectroscopy. NMR in Biomedicine 2016;29(4):400-410.
3. Novikov DS, Jensen JH, Helpern JA, Fieremans E. Revealing mesoscopic structural universality with diffusion. Proceedings of the National Academy of Sciences 2014;111(14):5088-5093.
4. Lee H-H, Papaioannou A, Novikov DS, Fieremans E. In vivo observation and biophysical interpretation of time-dependent diffusion in human cortical gray matter. NeuroImage 2020:117054.
5. Lee H-H, Papaioannou A, Kim S-L, Novikov DS, Fieremans E. A time-dependent diffusion MRI signature of axon caliber variations and beading. Communications Biology 2020;3(1):354.
6. Wu D, Martin LJ, Northington FJ, Zhang J. Oscillating gradient diffusion MRI reveals unique microstructural information in normal and hypoxia-ischemia injured mouse brains. Magnetic Resonance in Medicine 2014;72(5):1366-1374.
7. Does MD, Parsons EC, Gore JC. Oscillating gradient measurements of water diffusion in normal and globally ischemic rat brain. Magnetic Resonance in Medicine 2003;49(2):206-215.
8. Gong T, Tong Q, He H, Sun Y, Zhong J, Zhang H. MTE-NODDI: Multi-TE NODDI for disentangling non-T2-weighted signal fractions from compartment-specific T2 relaxation times. NeuroImage 2020;217:116906.
9. Veraart J, Novikov DS, Fieremans E. TE dependent Diffusion Imaging (TEdDI) distinguishes between compartmental T2 relaxation times. NeuroImage 2018;182:360-369.
10. Foo TKF, Tan ET, Vermilyea ME, Hua Y, Fiveland EW, Piel JE, Park K, Ricci J, Thompson PS, Graziani D, Conte G, Kagan A, Bai Y, Vasil C, Tarasek M, Yeo DTB, Snell F, Lee D, Dean A, DeMarco JK, Shih RY, Hood MN, Chae H, Ho VB. Highly efficient head-only magnetic field insert gradient coil for achieving simultaneous high gradient amplitude and slew rate at 3.0T (MAGNUS) for brain microstructure imaging. Magnetic Resonance in Medicine 2020;n/a(n/a).
11. Tan ET, Shih RY, Mitra J, Sprenger T, Hua Y, Bhushan C, Bernstein MA, McNab JA, DeMarco JK, Ho VB, Foo TKF. Oscillating diffusion-encoding with a high gradient-amplitude and high slew-rate head-only gradient for human brain imaging. Magnetic Resonance in Medicine 2020;n/a(n/a).
12. Arbabi A, Kai J, Khan AR, Baron CA. Diffusion dispersion imaging: Mapping oscillating gradient spin-echo frequency dependence in the human brain. Magnetic Resonance in Medicine 2019;n/a(n/a).
Figures