3393
WM motor learning can be detected using low frequency oscillations in time series functional MRI1Engineering Science, Simon Fraser University, Surrey, BC, Canada, 2Biomedical Physiology and Kinesiology, Simon Fraser University, Burnaby, BC, Canada, 3Psychology, University of Victoria, Victoria, BC, Canada, 4Computing Science, Simon Fraser University, Surrey, BC, Canada
Synopsis
A gap exists in developing a sensitive method for detecting functional neuroplasticity in white matter. To investigate this, participants trained on a motor learning task for two weeks and were scanned pre and post training using task based BOLD fMRI and DTI. Low frequency oscillations in time series BOLD data demonstrated that average amplitudes decreased with training in the internal capsule and corpus callosum genu. DTI analysis detected white matter neuroplasticity in internal capsule and corona radiata using fractional anisotropy. The distributed effect of motor learning suggests that multi-modal whole brain approaches will provide a more comprehensive understanding neuroplasticity.
INTRODUCTION
Multimodal MRI metrics including BOLD fMRI, myelin water imaging, diffusion tensor imaging (DTI), and cortical thickness have proven effective at detecting various brain changes as a result of motor-learning [1]–[4]. The use of MRI modalities for imaging neuroplasticity are prevalent in the literature, however, functional studies are dominated by gray matter (GM) findings. A gap exists in developing a robust method for detecting functional neuroplasticity in white matter (WM). Previous work using an WM-optimized hemodynamic response function (HRF) has detected a functional learning effect in the internal capsule [5]. Recent investigations have also confirmed that GM and WM hemodynamic responses take on characteristic profiles [6], [7] such that WM function may be poorly detected based on canonical HRFs. Low frequency oscillations (LFO) in BOLD signal represent intrinsic neural oscillations typically 0.01-0.4 Hz [8]. LFOs are believed to be responsible for recruiting and synchronizing brain networks and neural and cognitive processes [9]. They have been studied extensively in GM for the past 25 years, but have only recently been observed in specific WM structures [10], [11]. We hypothesized that functional neuroplasticity changes can be detected in key WM regions by characterizing the impact of motor learning on LFO. We also aim to link LFO and DTI results to confirm the finding.METHODS
Twelve healthy right hand-dominant participants were recruited. A detailed explanation of the experimental design and task may be found in Frizzell et al. 2020 [5]. In brief, participants trained on a visual-motor task for a few minutes each day. Each participant was scanned 3 times, at baseline, after one week of at home training, and at endpoint, after two weeks of at home training. The task required participants to trace a path displayed on a screen as rapidly and accurately as possible. The non-dominant hand data from baseline and endpoint scans were used. For each timepoint a T1 structural scan was acquired following task-based BOLD fMRI. DTI data were acquired using a single-shot EPI sequence with 32 diffusion directions and b-value of 800. fMRI data were preprocessed using FSL BET and FEAT functions following standard practices [12]. Motion correction was completed using the MCFLIRT function. The fMRI data were also slice time corrected and temporally high pass filtered with a cut-off of 100s. The DTI data were analyzed using FSL Diffusion Toolkit following standard practices [13]. The data were motion and eddy current corrected and tensors were calculated using FSL’s DTIFIT function. Track-based spatial statistics were used complete the pre-statistical analysis on fractional anisotropy (FA) maps. Regions of interest masks (ROI) were computed from significant clusters of fMRI change between baseline and endpoint from [5] and correlation based time series approach modified from Ding et al. 2013 [14]. This approach produced three WM ROIs within the internal capsule (IC), corpus callosum genu (CC), and superior corona radiata (CR). BOLD signal voxel intensity was averaged over the ROIs and were extracted for each TR as time series data. The data was demeaned and variance was normalized. The time series data was transformed to frequency-domain using MATLAB’s Fast Fourier Transform. Three frequency bands (band B: 0.01–0.08Hz; band C: 0.08–0.15Hz; band D: 0.15–0.22Hz) were examined. A heteroskedastic linear mixed-effects model was employed in R to estimate the effect of training between baseline and endpoint on amplitude in each frequency band, adjusted for correlation of repeated observations. Participant was set as the random-effect; whereas, ROI and frequency bands as fixed-effects. FSLs Randomise function was used to compute changes in DTI FA between baseline and endpoint.RESULTS
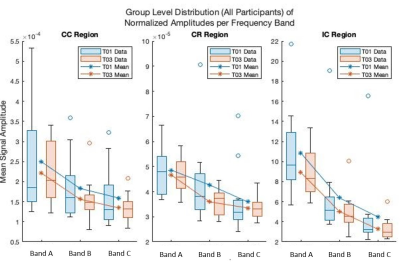
Figure 1 shows the average amplitudes within each frequency band for each ROI and timepoint. Table 1 shows the output of the linear mixed-effects model detecting a significant decrease in average amplitude within the frequency bands across the CC and IC. No significant effect was found for frequency band. Table 2 shows the FA results showed significant (p < 0.05, FWE) in the CR and IC.DISCUSSION
This frequency based physiological oscillations analysis focuses on the time series data to allow an investigation into WM functional neuroplasticity. The change of average amplitudes as a result of motor learning for the IC and CC ROIs regardless of frequency band provides additional data evidencing WM functional neuroplasticity, as previously reported using WM specific HRFs [5]. The result validates the hypothesis that LFOs are sensitive to motor learning effects in key WM tracts. The DTI analysis also detected WM structural neuroplasticity as an increase in FA in the IC and CR. While LFOs and DTI may not perfectly overlap in the regions with detected significant effects both show evidence of WM neuroplasticity. These results offer compelling evidence that neuroplasticity results in a distributed effect and that a multi-modal approach will net a more comprehensive model of brain changes. In future, employing other modalities such as myelin water imaging may help us better understand the structural effects that underpin the detected brain changes.Acknowledgements
This study received support from the Natural Sciences and Engineering Research Council Discovery Grant #206875 and from the Surrey Hospital and Outpatient Center Foundation under Grant FH2017-001.References
[1] M. V. Sale, L. B. Reid, L. Cocchi, A. M. Pagnozzi, S. E. Rose, and J. B. Mattingley, “Brain changes following four weeks of unimanual motor training: Evidence from behavior, neural stimulation, cortical thickness, and functional MRI,” Hum. Brain Mapp., vol. 38, no. 9, pp. 4773–4787, 2017.
[2] L. B. Reid, M. V. Sale, R. Cunnington, J. B. Mattingley, and S. E. Rose, “Brain changes following four weeks of unimanual motor training: Evidence from fMRI-guided diffusion MRI tractography,” Hum. Brain Mapp., vol. 38, no. 9, pp. 4302–4312, 2017.
[3] C. Sampaio-Baptista and H. Johansen-Berg, “White Matter Plasticity in the Adult Brain,” Neuron, vol. 96, no. 6, pp. 1239–1251, 2017.
[4] B. Lakhani et al., “Motor Skill Acquisition Promotes Human Brain Myelin Plasticity,” Neural Plast., vol. 2016, pp. 1–7, 2016.
[5] T. Frizzell, L. A. Grajauskas, C. C. Liu, S. Ghosh Hajra, X. Song, and R. C. N. D’Arcy, “White matter neuroplasticity: Motor learning activates the internal capsule and reduces hemodynamic response variability,” Fontiers Hum. Neurosci. (Manuscript Submitt. Publ., 2019.
[6] M. J. Courtemanche, C. Sparrey, X. Song, A. MacKay, and R. C. N. D’Arcy, “Detecting white matter activity using conventional 3 Tesla fMRI: An evaluation of standard field strength and hemodynamic response function,” Neuroimage, pp. 145–150, 2018.
[7] M. Li, A. T. Newton, A. W. Anderson, Z. Ding, and J. C. Gore, “Characterization of the hemodynamic response function in white matter tracts for event-related fMRI,” Nat. Commun., vol. 10, no. 1, p. 1140, Dec. 2019.
[8] B. Biswal, F. Zerrin Yetkin, V. M. Haughton, and J. S. Hyde, “Functional connectivity in the motor cortex of resting human brain using echo‐planar mri,” Magn. Reson. Med., vol. 34, no. 4, pp. 537–541, 1995.
[9] X. N. Zuo et al., “The oscillating brain: Complex and reliable,” Neuroimage, vol. 49, no. 2, pp. 1432–1445, Jan. 2010.
[10] Y. Jiang et al., “White-matter functional networks changes in patients with schizophrenia,” Neuroimage, vol. 190, no. November 2017, pp. 172–181, 2019.
[11] G. J. Ji, W. Liao, F. F. Chen, L. Zhang, and K. Wang, “Low-frequency blood oxygen level-dependent fluctuations in the brain white matter: more than just noise,” Sci. Bull., vol. 62, no. 9, pp. 656–657, May 2017.
[12] M. W. Woolrich, B. D. Ripley, M. Brady, and S. M. Smith, “Temporal Autocorrelation in Univariate Linear Modeling of FMRI Data,” Neuroimage, vol. 14, no. 2001, pp. 1370–1386, 2001.
[13] S. M. Smith et al., “Advances in Functional and Structural MR Image Analysis and Implementation as FSL Technical Report TR04SS2,” 2004.
[14] Z. Ding, A. T. Newton, R. Xu, A. W. Anderson, V. L. Morgan, and J. C. Gore, “Spatio-temporal correlation tensors reveal functional structure in human brain,” PLoS One, vol. 8, no. 12, 2013.
Figures