3392
Dynamic neurometabolic and functional changes in the dorsolateral prefrontal cortex in a working memory: a combined 1H fMRS and fMRI study1Division of Clinical Neuroscience, School of Medicine, University of Nottingham, Nottingham, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3NIHR Nottingham Biomedical Research Centre, Queen’s Medical Centre, University of Nottingham, Nottingham, United Kingdom, 4School of Psychology, University of Nottingham, Nottingham, United Kingdom
Synopsis
Dynamic changes in Glx [glutamate + glutamine] and GABA concentrations were investigated in the dorsolateral prefrontal cortex (DLPFC) during a working memory using fMRS and fMRI at 3T. During the 12 minutes 2-back task Glx showed the trend of increase, while GABA levels remained stable. fMRI confirmed the increased regional activity in the DLPFC during 2-back vs. 0-back task. Importantly, the Glx changes were correlated with the DLPFC BOLD regional activity and task performance during 2-back task. Our results suggest that working memory related Glx changes may be involved in the DLPFC’s functional response and performance in working memory.
Introduction
The dorsolateral prefrontal cortex (DLPFC) is one of the key regions in executive function including working memory. Neural activity in the brain is associated with the balance between glutamate and GABA, which are excitatory and inhibitory neurotransmitters, respectively. Neurometabolic changes in response to visual stimulation have been widely investigated using functional MRS (fMRS) in the occipital lobe, where glutamate increases have been reported1,2. A recent study3 investigated changes in combined glutamate and glutamine (Glx) levels within the DLPFC during a 2-back task and showed increased Glx relative to baseline. However, the neurochemical mechanisms reflected in the excitatory/inhibitory balance (EIB) that underpin working memory function in the DLPFC remain unclear. Here, we investigated dynamic changes in Glx, GABA, and EIB levels during working memory processing using a combined fMRS with fMRI. We hypothesized that working memory would modulate Glx and GABA levels, thereby increasing the EIB, and that the dynamic changes in these neurometabolites would be associated with fMRI-measured signal changes in the DLPFC as well as task performance.Methods
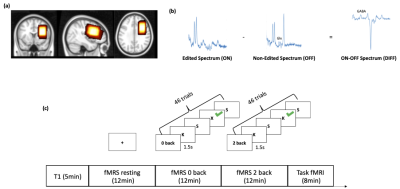
12 healthy participants (9 Females) were recruited (25±2 years). A 3 Tesla GE MR imaging system was used to acquire T1-weighted images, spectra with MEGA-PRESS (TR/TE=2000ms/68ms, editing-frequency = 1.9 ppm) and BOLD-fMRI images. For the fMRS, 160 ON and OFF spectra were acquired from a 40x25x30mm voxel, placed over the left DLPFC (Figure 1a). For functional imaging, an EPI sequence was used (TR/TE=2000/20ms, slice thickness=3mm, 40 axial slices). During both fMRS and fMRI, participants performed a 2-back letter memory task and a 0-back task as a control. In the fMRS, there were 3 sessions including rest, 0-back and 2-back task. A fixation-cross was presented during resting. The 0-back and 2-back tasks consisted of seven blocks with 8s-long rest periods between each block. Raw spectra were acquired, coil-combined and averaged in blocks of 20 spectra, such that 8 time points were included in each session. Metabolite spectra were fit with LCModel and metabolite changes were calculated relative to the first time point of each participant. For fMRI, 0 back and 2 back tasks were interleaved. 18 trials were included in a block of 0-back and 2-back both, and the tasks consisted of six blocks. (Figure 1c).Results
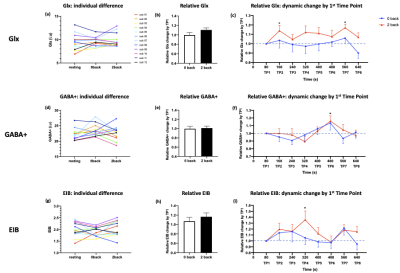
An increase in Glx was found at second time point (TP2) (t = 2.417, p = 0.034, uncorrected) and TP7 (t = 4.393, p = 0.001) compared to TP1 (Figure 2c). The averaged relative Glx levels were nominally larger during the 2-back compared to 0-back tasks (t = 1.574, p = 0.144). (Figure 2b)GABA showed increase at a single time point TP6 (t=2.414, p=0.034) during 2-back task (Figure 2f), but the averaged relative GABA was similar between 2-back and 0-back (t = 0.220, p = 0.830). (Figure 2e)
An increase in EIB at TP4 was seen (t = 2.387, p = 0.036) while the averaged relative EIB was not significantly different (t = 0.912, p = 0.381). (Figure 2h)
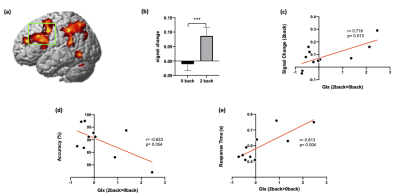
fMRI results demonstrated that 2-back task induced activation in the DLPFC and parietal cortex (Figure 3a). Region of interest analysis with fMRS voxel revealed that 2-back task significantly increased regional activity in the DLPFC compared to 0-back task (t = 5.234, p < 0.001) (Figure 3b). There was a significant positive correlation between the Glx changes (2-back > 0-back) and DLPFC activity during 2-back task (r = 0.718, p = 0.013) (Figure 3c). 2-back task modulated Glx changes relative to 0-back were also correlated with 2-back task performance (accuracy: r = -0.623, p = 0.054, RT: r = 0.813, p = 0.004) (Figure 3d & e).
Discussion
The goal of the present study was to investigate dynamic neurochemistry in the DLPFC during working memory function. Our fMRS and fMRI approach demonstrated that 2-back working memory task increased Glx at several time points and increased regional activity in the DLPFC. Importantly, task-modulated Glx changes (2-back > 0-back) were significantly associated with task-induced regional activity in the DLPFC during 2-back task. In preclinical studies, it was reported that glutamate-glutamine neurotransmitter cycling rate and glucose metabolic rate were strongly connected4. Consistent with previous findings, our results indicate that increased Glx in the DLPFC may reflect increased excitatory neurotransmitters or increased glucose energy metabolism as the task demand increases. Interestingly, we found a significant relationship between task-modulated Glx changes and task performance. Individuals with increased Glx during 2-back task showed a poorer task performance with slower response time. This suggests that increased Glx along with upregulated regional activity in the DLPFC does not contribute to efficient working memory processing but may indicate failing compensatory mechanisms.Conclusion
Dynamic changes of Glx levels during working memory suggests a task dependent DLPFC modulation of metabolic activity related to glucose consumption and excitatory neurotransmitters. The direct correlation between Glx changes and fMRI measured regional activity changes in the DLPFC, but anticorrelation with task performance further suggest increased prefrontal neurometabolic response as marker impaired working memory processing.Acknowledgements
We appreciate the support from Andrew Cooper and Jan Paul for MRI scanner operation.References
1. Lin Y, Stephenson MC, Xin L, Napolitano A, Morris PG. Investigating the metabolic changes due to visual stimulation using functional proton magnetic resonance spectroscopy at 7 T. J Cereb Blood Flow Metab (2012) 32(8): 1484-95
2. Bednarik P, Tkac I, Giove F, DiNuzzo M, Deelchand DK, Emir UE, Eberly LE, Mangia S. Neurochemical and BOLD responses during neuronal activation measured in the human visual cortex at 7 Tesla. J Cereb Blood Flow Metab (2015) 35(4): 601-10
3. Woodcock EA, Anand C, Khatib D, Diwadkar VA, Stanley JA (2018) Working Memory Modulates Glutamate Levels in the Dorsolateral Prefrontal Cortex during 1H fMRS. Front. Psychiatry 9:66
4. Sonnay S, Duarte JM, Just N, Gruetter R. Compartmentalised energy meta- bolism supporting glutamatergic neurotransmission in response to increased activity in the rat cerebral cortex: a 13C MRS study in vivo at 14.1 T. J Cereb Blood Flow Metab (2016) 36:928–40
Figures