3377
New Frontiers of Human Neuroscience: 0.5 mm isotropic 7T in Vivo Human fMRI1CMRR, University of Minnesota, minneapolis, MN, United States, 2Department of Neurosurgery, University Of Minnesota, minneapolis, MN, United States
Synopsis
The goal of 0.1μL voxel resolution for human fMRI set by the BRAIN Initiative has thus far been nearly impossible to reach, due to the overwhelming thermal noise contribution at such high spatial resolutions. Here we show that, with the recently developed denoising algorithm NORDIC, we are able to achieve robust functional activation to visual stimuli at 0.5 mm isotropic (0.125μL) voxel resolution at 7T field strengths with only 20 minutes of data, a results that is unobtainable with conventional data reconstruction. Preliminary layer findings show a central peak, suggesting that this may dramatically improve quantification of depth-dependent activation profiles.
Introduction
Shortly after the discovery of Blood-Oxygen-Level-Dependent (BOLD) fMRI for human brain studies, the introduction of ultra-high fields (UHF) (≥7 Tesla) (1) and more efficient and highly accelerated acquisition protocols targeting high resolution UHF fMRI applications (2) enabled development of submillimeter resolution functional mapping in the human brain; these studies were motivated by the ability to study mesoscopic scale organizations, such as orientations domains (3), which, prior to the successes of fMRI, could only be addressed by invasive electrophysiology and optical techniques in animal model systems. Today, 7T fMRI studies in the human brain at this scale is a flourishing research topic (4,5). However, at typical ~0.8 mm isotropic resolutions achieved to date, these submillimeter fMRI data are highly SNR starved and marginally adequate for accurately mapping mesoscopic scale organizations (6). This limitation is reflected in the first strategic plan of the BRAIN Initiative Working group, which challenged the MR community to achieve whole brain imaging studies with 0.1 μL or smaller voxel volumes (7,8). Recently, we employed a novel denoising algorithm, NORDIC, to suppress thermal noise, the dominant noise source on high-resolution fMRI, to improve tSNR and BOLD response estimations, without loss of spatial precision. Here we utilize this denoising strategy, providing the first ever demonstration of in vivo human 7T Gradient echo (GE) BOLD fMRI with 0.5 mm isotropic voxels (0.125 μL) obtained in relatively short data collection times (5 to 20 min).Methods
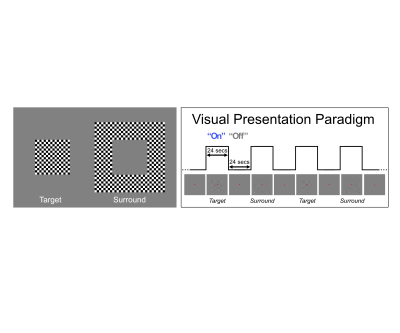
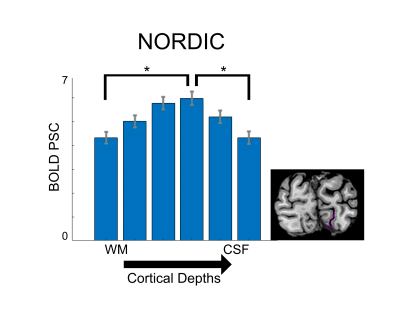
Functional MRI data were collected with a 7T Siemens System with a single transmit, 32-channel receive NOVA head coil. T2*-weighted images were collected using a 3D GE EPI protocol (40 slices, TR: 83s, VAT: 3652ms, iPAT: 3, 6/8ths Partial Fourier, 0.5mm isotropic voxels, TE: 26.4ms, Bandwidth: 820Hz). For each participant, shimming to improve B0 homogeneity over occipital regions was conducted manually. Functional preprocessing, which included 3D rigid body motion correction and low drift removals, was kept at a minimum and constant across reconstructions. The experimental procedure consisted of a standard 24 seconds on 24 seconds off block design paradigm in which participants viewed a center (i.e. target) and a surround square checkerboard counterphase flickering (at 6 Hz) gratings, subtending approximately 6.5 degrees of visual angle. Each run lasted just 5 minutes (3 trials x 2 conditions). Within V1, we parcellated the cortex into 6 equivolume cortical depths, ranging from 10% to 90% distance from the pial surface and analyzed the activity elicited by the target condition within the retinotopic representation of the target.Results
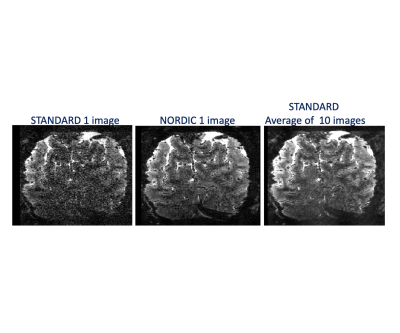
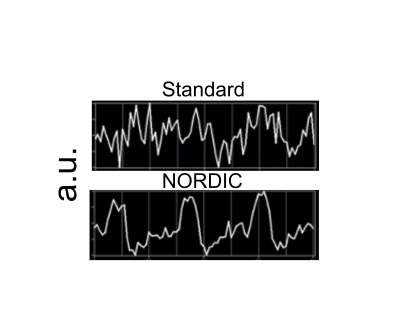
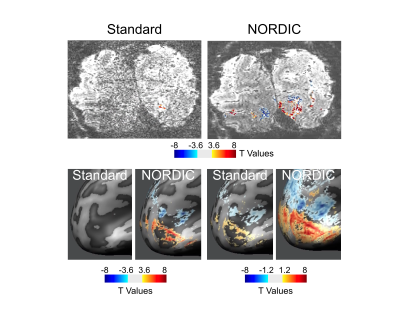
Image quality: Figure 1 illustrates a single slice at a single time point from a 0.5 mm isotropic resolution fMRI volume showing a dramatic improvement in image SNR following NORDIC denoising. Ten images of standard reconstruction are required to achieve image SNR comparable to that obtainable in a single NORDIC image. Careful examinations of these images also illustrate a lack of spatial blurring by NORDIC, consistently with (9). BOLD activation Time-course: Figure 2 shows the time-courses of a single voxel medially located within the V1 representation of the target stimulus (standard (top) and NORDIC (bottom)). Contrary to the standard time-course, which is dominated by noise, the NORDIC time-course displays all expected temporal characteristics of BOLD responses to the 3 presentations of the target stimulus. Activation Maps: t-maps computed for the target>surround contrast for 4 concatenated 5 min runs show a significantly (p<.01 corrected) larger extent of activation for NORDIC than Standard images. Cortical depths analyses: for the NORDIC data, BOLD percent signal change amplitude peaked in the middle depth, which was significantly larger (p<0.05 corrected) than in outer and inner depths; for the Standard reconstruction, no significant BOLD activation was found to be larger than 0 across all layers. These results are promising, but need to be verified across multiple subjects.Discussion
Processed with the Standard reconstruction, 0.5 mm isotropic resolution, 7T human fMRI data yield extremely noisy images, which are unusable for functional mapping. This is anticipated since 0.8 mm isotropic fMRI data has only marginally adequate SNR for functional mapping and going to 0.5 mm isotropic volume reduces the voxel volume, hence available signal per voxel, by a factor of 4.1. Consequently, statistically significant stimulus induced signal changes were simply not detected in the Standard reconstruction in the 0.5 mm isotropic resolution (0.125 µL voxel volume) data, but became robustly detectable with the use of NORDIC, allowing measurement of layer specific functional responses at this high resolution.Conclusion
We demonstrate the feasibility of functional mapping in the human brain at the resolution set as the first target in the BRAIN Initiative in order to achieve transformative changes in human brain imaging so as to “measure the wiring diagram and functional activity of the human brain at multiple scales—neuronal ensembles, circuits, and larger scale networks (‘circuits of circuits’) (7,8). This was achieved by using a novel denoising algorithm to suppress thermal noise in the fMRI time series, the dominant noise source at such ultrahigh resolution imaging without blurring the images. This demonstration, especially together with ongoing instrumental developments such as new ultrahigh field instruments operating above 10 Tesla, heralds in the start of a major expansion in the frontiers of fMRI applications to neuroscience.Acknowledgements
This work was supported by NIH grants U01EB025144 (K.U.), P41 EB027061 (K.U.), P30 NS076408 (K.U.) and RF1 MH116978 (E.Y.)References
1. K. Ugurbil, Imaging at ultrahigh magnetic fields: History, challenges, and solutions. Neuroimage 168, 7-32 (2018).
2. S. Moeller et al., Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 63, 1144-1153 (2010).
3. E. Yacoub, K. Uludag, K. Ugurbil, N. Harel, Decreases in ADC observed in tissue areas during activation in the cat visual cortex at 9.4 T using high diffusion sensitization. Magnetic Resonance Imaging 26, 889-896 (2008).
4. F. De Martino et al., The impact of ultra-high field MRI on cognitive and computational neuroimaging. Neuroimage 168, 366-382 (2018).
5. S. O. Dumoulin, A. Fracasso, W. van der Zwaag, J. C. W. Siero, N. Petridou, Ultra-high field MRI: Advancing systems neuroscience towards mesoscopic human brain function. Neuroimage 168, 345-357 (2018).
6. L. Vizioli et al., Multivoxel Pattern of Blood Oxygen Level Dependent Activity can be sensitive to stimulus specific fine scale responses. Sci Rep 10, 7565 (2020).
7. L. A. Jorgenson et al., The BRAIN Initiative: developing technology to catalyse neuroscience discovery. Philos Trans R Soc Lond B Biol Sci 370 (2015).
8. BRAIN Initiative Working Group (2014) BRAIN 2025: A Scientific Vision.
9. L. Vizioli et al., A Paradigm Change in Functional Brain Mapping: Suppressing the Thermal Noise in fMRI. bioRxiv 10.1101/2020.11.04.368357 (2020).
Figures