3376
Mapping of Whole-brain Resting-State Networks with Half-millimetre Resolution using TR-external EPIK at 7T
Seong Dae Dae Yun1, Patricia Pais-Roldán1, Nicola Palomero-Gallagher2,3,4, and N. Jon Shah1,5,6,7
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 1, INM-1, Forschungszentrum Juelich, Juelich, Germany, 3C. & O. Vogt Institute for Brain Research, Heinrich-Heine-University, Duesseldorf, Germany, 4Department of Psychiatry, Psychotherapy and Psychosomatics, Medical Faculty, RWTH Aachen, Aachen, Germany, 5Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 6JARA - BRAIN - Translational Medicine, Aachen, Germany, 7Department of Neurology, RWTH Aachen University, Aachen, Germany
1Institute of Neuroscience and Medicine 4, INM-4, Forschungszentrum Juelich, Juelich, Germany, 2Institute of Neuroscience and Medicine 1, INM-1, Forschungszentrum Juelich, Juelich, Germany, 3C. & O. Vogt Institute for Brain Research, Heinrich-Heine-University, Duesseldorf, Germany, 4Department of Psychiatry, Psychotherapy and Psychosomatics, Medical Faculty, RWTH Aachen, Aachen, Germany, 5Institute of Neuroscience and Medicine 11, INM-11, JARA, Forschungszentrum Juelich, Juelich, Germany, 6JARA - BRAIN - Translational Medicine, Aachen, Germany, 7Department of Neurology, RWTH Aachen University, Aachen, Germany
Synopsis
Mapping of resting-state networks has been performed in numerous studies to investigate overall brain function and its underlying connectivity. However, attempts to acquire resting-state fMRI data with high spatial resolution have been hampered by the current technical limitations. The spatial resolution from recent submillimetre-resolution fMRI studies still remains around 0.7 × 0.7 × 0.7 mm3, with only partial brain coverage. This work employs a novel high-resolution fMRI method, TR-external EPIK, to perform resting-state fMRI with half-millimetre in-plane resolution and whole-brain coverage at 7T. Various resting-state networks over the whole-brain have been identified with high mapping fidelity onto the cortical ribbon.
Introduction
Resting-state fMRI is of great interest in the fMRI community since correlated brain areas (i.e. resting-state networks) can be identified based on the brain’s overall activity, while task-evoked fMRI reflects only a small fraction of the brain function.1,2 Therefore, whole-brain coverage is often a requirement for resting-state fMRI. Recent advances in fMRI techniques allow the acquisition of functional signals with a submillimetre voxel size, enabling identification of cortical depth-dependent functional activation. However, most previous submillimetre-resolution fMRI studies have investigated the depth-dependent activation for a particular brain region as evoked by a task paradigm.3-7 This is mainly due to the technical limitation of current fMRI methods where spatial resolution still remains around 0.7 × 0.7 × 0.7 mm3 with only partial brain coverage. To overcome this limitation, a novel fMRI method, TR-external EPIK, was presented in our earlier work, and its application to finger-tapping fMRI was demonstrated.8,9 This work aims to illustrate its use for mapping of resting-state networks with half-millimetre in-plane resolution and whole-brain coverage at 7T.Methods
Resting-state fMRI data were acquired with a half-millimetre protocol implemented using TR-external EPIK. The imaging parameters were as follows: TR/TE = 3500/22 ms, FOV = 210 × 210 mm2, matrix = 408 × 408 × 108 slices (0.51 × 0.51 × 1.0 mm3), FA = 85° and 172 temporal volumes (i.e. 10-minutes of resting-state fMRI data). In order to regress out the effect of physiological noise, a pneumatic belt and pulse-oximeter were used to record the respiratory and cardiac signals of healthy subjects who were screened with a standard safety procedure. The above study protocol was approved by the local institutional review board (RWTH Aachen University, Germany) and was employed on a Siemens Magnetom Terra 7T scanner with a single-channel transmit/32-channel receive Nova medical head coil. The resting-state data were pre-processed using AFNI (NIH, Bethesda Maryland, USA), and included the following steps: slice-timing correction, realignment, regression of cardio-respiratory signals and temporal filtering with regression of motion parameters. In addition, a phase-based correction method was also applied to reduce the effect of large-vessel blood-oxygenation-level-dependent (BOLD) effect, typically seen in the gradient-echo sequences.10,11 Following the above pre-processing, independent component analysis (ICA) was carried out with 80 components using Melodic in FSL (FMRIB, Oxford, UK).Results
Figure 1 shows reconstructed images obtained from TR-external EPIK. Three representative slices chosen from the 108 slices are displayed in Fig. 1a. Here, magnified views with detailed spatial representation of anatomical structures for specific brain regions (marked by r1~r4) are also shown in Fig. 1b. Figure 1c shows the coronal and sagittal presentation of the entire 108 slices, in which the complete extent of the brain covered by the imaging protocol can be verified. Moreover, the cortical ribbon can also be clearly observed in these resliced images. Figure 2 shows mapping results of six representative resting-state networks (dorsal-DMN, ventral-DMN, visual, sensorimotor (LH: Left Hemisphere), sensorimotor (RH: Right Hemisphere) and fronto-parietal). The activated voxels were identified with a statistical threshold (probability ≥ 0.7) and directly overlaid on a re-aligned functional scan. The mapping results are displayed in three different sectional views (axial, coronal and sagittal), demonstrating the simultaneous detection of the six resting-state networks distributed throughout the brain from a single fMRI session. Moreover, a high mapping fidelity onto the cortical ribbon was also verified and was mainly attributed to the half-millimetre in-plane voxel size. For a more detailed visual inspection, each resting-state network is displayed separately in its representative slice location (see Fig. 3). Here, one can also see that the voxels associated with resting-state networks are highly localised along the cortical ribbon. For three of the identified networks (i.e. dorsal-DMN, sensorimotor (RH) and visual), a specific ROI was selected (see the green rectangle in Fig. 3), and its enlarged depiction is shown in Fig. 4a. Here, a line crossing the cortical ribbon (see a black line marked with P1 and P2) was manually defined for each ROI, and the BOLD signal profile along this line was examined. The obtained BOLD signal profiles are depicted as a solid black line in Fig. 4b. To aid visual inspection, the grey matter (GM) region obtained from a standard segmentation routine in FSL is also shown as a blue dotted line. In addition, the signal of the background images along the line is delineated with a black dotted line. This figure shows that the behaviour of resting-state BOLD signals was characterised along the cortical depth and that the BOLD activation profile is almost confined to the GM regions.Discussion and conclusions
This work demonstrates the application of TR-external EPIK to whole-brain, half-millimetre resolution mapping of resting-state networks at 7T. The imaging voxel (0.51 × 0.51 × 1.0 mm3; 0.26 mm3) enabled the detection of various resting-state networks distributed throughout the brain from a single fMRI measurement, with a high mapping fidelity onto the grey matter. Mapping of resting-state networks with the spatial resolution and brain coverage provided here has not been previously achieved. The slice thickness (1 mm) was not reduced further to ensure a reasonable SNR for fMRI. However, an even smaller voxel size may be possible with TR-external EPIK by compromising imaging parameters.Acknowledgements
No acknowledgement found.References
- Smitha KA, Akhil Raja K, Arun KM, et al. Resting state fMRI: A review on methods in resting state connectivity analysis and resting state networks. Neuroradiol J. 2017;30(4):305-17.
- van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20(8):519-34.
- Guidi M, Huber L, Lampe L, Gauthier CJ, Moller HE. Lamina-dependent calibrated BOLD response in human primary motor cortex. Neuroimage. 2016;141:250-61.
- Chai YH, Li LQ, Huber L, Poser BA, Bandettini PA. Integrated VASO and perfusion contrast: A new tool for laminar functional MRI. Neuroimage. 2020;207.
- Huber L, Goense J, Kennerley AJ, et al. Cortical lamina-dependent blood volume changes in human brain at 7 T. Neuroimage. 2015;107:23-33.
- Kashyap S, Ivanov D, Havlicek M, Sengupta S, Poser BA, Uludag K. Resolving laminar activation in human V1 using ultra-high spatial resolution fMRI at 7T. Sci Rep-Uk. 2018;8.
- van Mourik T, van der Eerden JPJM, Bazin PL, Norris DG. Laminar signal extraction over extended cortical areas by means of a spatial GLM. Plos One. 2019;14(3).
- Yun S, Shah NJ. Full-FOV, Whole-brain, Half-millimetre Resolution fMRI at 7T using Accelerated multi-band EPIK with TR-external Phase Correction. In Proceedings of the 27th Annual Meeting of ISMRM, Montreal, Canada, 2019. Abstract 1167.
- Yun S, Pais-Roldán P, Shah NJ. Detection of Cortical Depth-dependent Functional Activation using Whole-brain, Half-millimetre Resolution EPIK at 7T. In Proceedings of the 28th Annual Meeting of ISMRM, 2020. Abstract 3863.
- Curtis AT, Hutchison RM, Menon RS. Phase based venous suppression in resting-state BOLD GE-fMRI. Neuroimage. 2014;100:51-9.
- Rua C, Costagli M, Symms MR, Biagi L, Donatelli G, Cosottini M, et al. Characterization of high-resolution Gradient Echo and Spin Echo EPI for fMRI in the human visual cortex at 7T. Magn Reson Imaging. 2017;40:98-108.
Figures
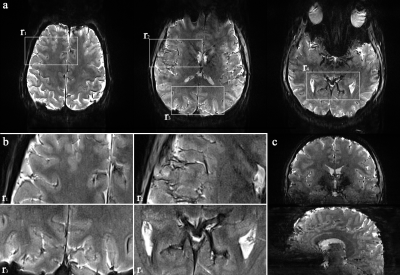
Figure 1. (a) Three representative axial slices, selected from 108 slices, (b) enlarged depiction of the selected
ROIs (r1 ~ r4) and (c)
coronal and sagittal views of the 108 axial slices. This figure depicts a detailed
spatial representation of the anatomical structures and demonstrates the brain
coverage provided by TR-external EPIK.
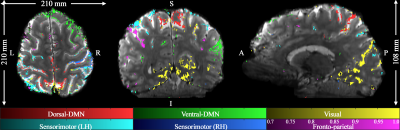
Figure 2. Mapping of six
representative resting-state networks with the half-millimetre protocol (0.51 ×
0.51 × 1.0 mm3) is shown: dorsal-DMN, ventral-DMN, visual,
sensorimotor (LH: Left Hemisphere), sensorimotor (RH: Right Hemisphere) and
fronto-parietal; DMN denote the default mode network. The results, depicted in
three sectional views (axial, coronal and sagittal) above, effectively
demonstrate the brain coverage provided by TR-external EPIK (210 × 210 × 108 mm3)
as well as the localisation of the functional voxels on the cortical ribbon.
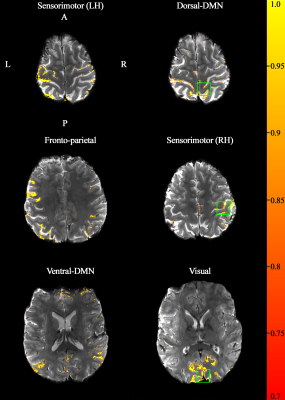
Figure 3. Each of the six
resting-state networks is shown on a representative slice. The significance of
the functional activation is coded with a red-to-yellow scale (see the
probability range in the colour-bar on the right side). It can be clearly seen
that the activated voxels are locally distributed along the cortical ribbon.
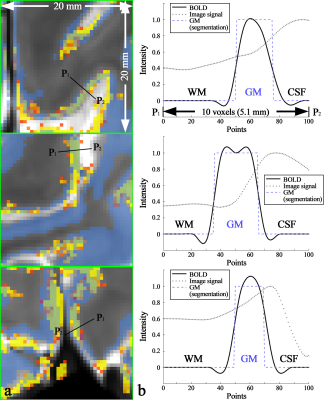
Figure 4. (a) Enlarged depiction of the ROIs marked by the green rectangles in
the right column of Fig. 3 and (b)
line profile of BOLD signals sampled along the black line, denoted with the
points P1 (start) and P2 (end) in the image panel a. The
examined line length was 5.1 mm (10 voxels) and 100 points are equidistantly
re-sampled on this line. The image panel b demonstrates that the resting-state
activation was characterised along the cortical depth for the three networks (dorsal-DMN,
sensorimotor (RH) and visual) and that they are almost confined to the GM
region.